Chief Complaint: Double vision, bulging eyes and drooping of the left upper eyelid
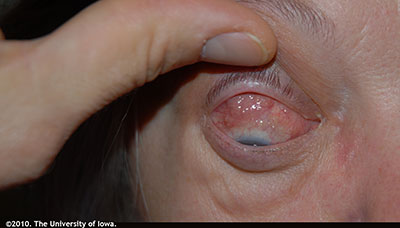
History of Present Illness: A 56-year-old male patient presented to the oculoplastics clinic reporting a two-month history of progressive proptosis and drooping of the left upper eyelid. He had also been experiencing binocular diplopia for several weeks. He had constant generalized redness around the eye for several years that was ascribed to chronic seasonal allergies. Furthermore, over the last several months, he had noticed that his eyes began to bulge (on the left side more than the right), and he began experiencing pain around the left eye. He did not describe any other changes in his vision aside from the diplopia and proptosis.
Medical History:
Past Ocular History: Strabismus, status post surgical correction at the age of 3
Medications: Dexamethasone 1mg BID, albuterol, fluticasone, fluticasone/salmeterol, doxepin
Allergies: NKDA.
Family History:
Social History: Patient denies tobacco or alcohol use. The patient is an attorney and is married, without children. There is no recent or remote history of travel outside of the United States.
Visual Acuity: 20/25 OD; 20/25 OS
Pupils: Normal size and reaction OU. No relative afferent pupillary defect OU
Motility: Normal OD, Mild abduction deficit OS
Visual fields: Full to confrontation OU
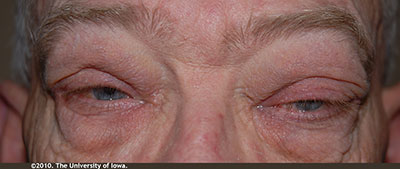
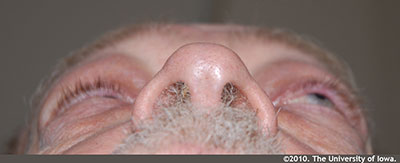
External exam: Erythematous and flaky periorbital skin OU. Mild periorbital edema OU. (Figure 1); Left sided proptosis (Figure 2)
Slit Lamp Exam:
Dilated Fundus Exam:
 |
 |
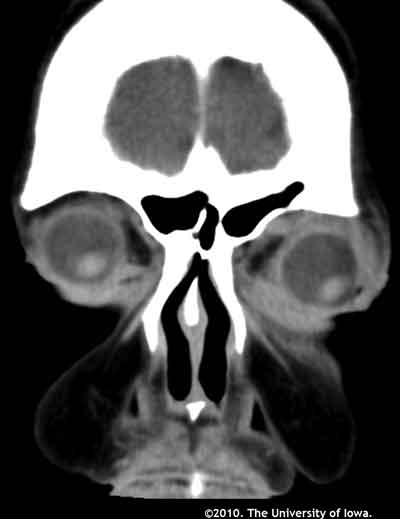
As part of the patient's workup for what appeared to be bilateral orbital inflammation, an orbital CT was performed and a complete blood count was obtained.
 |
As part of his workup for eosinophilia, the patient underwent a bone marrow biopsy that demonstrated an abnormally high number of eosinophils. The bone marrow was otherwise normal with no evidence of other blood dyscrasias.
Given that the etiology of the inflammation and eosinophilia was unclear, a left anterior orbitotomy was performed and a biopsy of orbital mass was obtained.
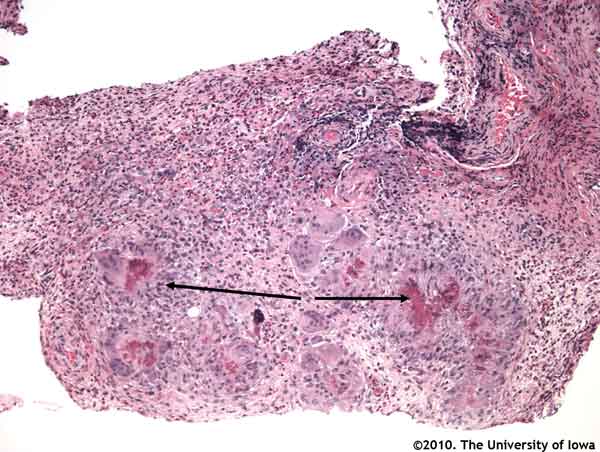
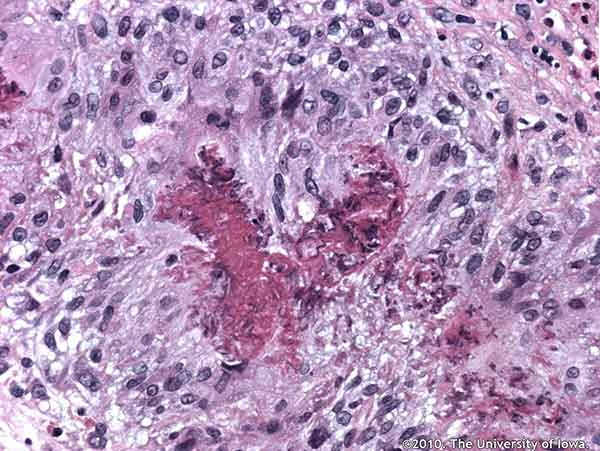
Histopathologic analysis of the biopsied tissue revealed an inflammatory infiltrate in the orbital fat composed of numerous eosinophils, epithelioid histiocytes, lymphocytes, and occasional multinucleated giant cells. Focal areas of necrosis were present in some of the granulomatous areas. In some areas, inflammatory cells surrounded small and medium-sized blood vessels. Periodic acid Schiff (PAS), Ziehl-Neelsen, Gomori methenamine silver (GMS), Warthin-Starry, Giemsa, Gram and Fite-Faraco stain were all negative for organisms.
 |
 |
The patient's constellation of clinical and laboratory findings satisfied the American College of Rheumatology criteria for Churg-Strauss Syndrome (CSS) (Table 1) Specifically, his asthma, peripheral eosinophilia, paranasal sinusitis and histologic proof of eosinophilic perivasculitis made CSS the leading diagnosis for this patient.
The patient was referred to rheumatology and was treated with high-dose corticosteroids. He reported complete resolution of his symptoms after a few weeks of treatment. At one year, he remained symptom-free, but required a daily dose of steroid to keep his symptoms under control. The managing rheumatologist is considering transitioning the patient to a steroid-sparing agent for long-term disease control.
Churg-Strauss syndrome (CSS) was first described by Churg and Strauss in 1951 as a small and medium-vessel vasculitis characterized by asthma, hypereosinophilia and multi-system vasculitis (Sehgal, 1995). CSS is a rare disorder with an incidence of 1.3 to 6.8 cases per 1 million patients per year (Scott, 2000). The mean age of presentation is approximately 50 years, and there appears to be a slight male predominance.
There are three distinct clinical phases of CSS. The disease begins with asthma and atopic allergies that may begin in childhood. The first phase may be present for months to years before progression to the second, eosinophilic phase, which is characterized by eosinophilic infiltration with granulomatous inflammation and can have an array of clinical presentations, including granulomatous pneumonia, gastroenteritis, or, as in this case, orbital pseudotumor. The final third phase of the disease is the vasculitic phase, which typically involves small- and medium-sized vessels of the eyes, skin, gastrointestinal tract and heart. Coronary arteritis and myocarditis are the primary causes of morbidity and mortality (Noth, 2003). These phases do not always occur in a step-wise fashion, making the diagnosis a challenging one to make definitively. Patients are often treated for multiple distinct conditions before the unifying diagnosis of CSS is made.
The classic histologic findings in CSS are necrotizing arteritis, eosinophilic infiltration and extravascular granulomas. Our patient demonstrated a peri-arteritis along with eosinophilic infiltration and extravascular granulomas in both the orbit and in his bone marrow.
 |
The ophthalmic manifestations of CSS are varied and can result from granulomatous inflammation, vasculitis or both. Numerous neuro-ophthalmic manifestations of CSS have been reported, including cranial neuropathies, amaurotic episodes, arteritic ischemic optic neuropathy, and orbital pseudotumor. Vascular involvement can also present as a central or branch retinal artery occlusion secondary to vasculitis. Anterior segment involvement can present as conjunctivitis, scleritis, or keratouveitis (Golnik, 2004). Chronic conjunctivitis and conjunctival granulomas have also been reported (Figure 6).
Establishing a diagnosis can be difficult given the phasic nature and the often prolonged and sequential development of clinical and pathological features. CSS is distinguished from other granulomatous, vasculitic and eosinophilic syndromes by a clinical history that includes asthma, eosinophilia and rhinosinusitis, as well as other characteristic signs and symptoms (Table 2). Establishing a diagnosis depends on the presence of a constellation of findings, rather than any single feature. When the diagnosis was first described by Churg and Strauss in 1951, all patients examined had the following three findings: blood and tissue eosinophilia, necrotizing vasculitis and necrotizing granulomas centered on necrotic eosinophils (Churg, 1951). However, finding all three features in any single patient is rare and cannot be relied upon for diagnosis. The American College of Rheumatology set forth the most widely utilized criteria for diagnosing and classifying CSS; the presence of 4 or more criteria yields a sensitivity of 85% and a specificity of 99.7% (Table 1) (Masi, 1990).
CSS is typically treated with systemic steroids alone. However, in 20% of patients, cytotoxic drugs are required to stop progression of the disease. Major life-threatening organ involvement may require emergent treatment with pulse doses of intravenous corticosteroids combined with other cytotoxic medications (Grau, 2008).
|
The presence of 4 or more criteria yields a sensitivity of 85% and a specificity of 99.7%
| CSS | WG | MPA | HES | |
|---|---|---|---|---|
| Asthma | Yes | No | No | No |
| Eosinophilia | Yes | No | No | Yes |
| Rhinosinusitis | Yes | No | No | No |
| Lung involvement | Yes | Yes | Yes | Yes |
| Skin involvement | Yes | Yes | Yes | Yes |
| Heart involvement | Yes | Rare | Rare | Yes |
| ANCA positivity | Yes (40%, myelo-peroxidase) | Yes (90%, proteinase 3) | Yes (80%, myelo-peroxidase) | No |
| Vasculitis | Yes | Yes | Yes | No |
| Eosinophilic-rich infiltrate | Yes | No | No | Yes |
| Granuloma | Yes | Yes | No | No |
Abbreviations: Churg-Strauss Syndrome (CSS), Wegener's granulomatosis (WG), microscopic polyangiitis (MPA), hypereosinophilic syndrome (HES).
Epidemiology
|
Signs
|
Symptoms
|
Treatment
|
Churg J, Strauss L. Allergic granulomatosis, allergic angiitis, and periarteritis nodosa. Am J Pathol 1951; 27: 277-301.
Grau RG. Churg-Strauss syndrome: 2005-2008 update. Curr Rheumatol Rep 2008; 10: 453-8.
Masi AT, Hunder GG, Lie JT, Michel BA, Bloch DA, Arend WP, Calabrese LH, Edworthy SM, Fauci AS, Leavitt RY, et al. The American College of Rheumatology 1990 criteria for the classification of Churg-Strauss syndrome (allergic granulomatosis and angiitis). Arthritis Rheum 1990; 33:1094-100.
Noth I, Strek ME, Leff AR. Churg-Strauss syndrome. Lancet 2003; 361; 587-94.
Scott DG, Watts RA. Systemic vasculitis: epidemiology, classification and environmental factors. Ann Rheum Dis 2000; 59: 161-3.
Sehgal M, Swanson JW, DeRemee RA, Colby TV. Neurologial Manifestations of Churg-Strauss Syndrome. Mayo Clin Proc. 1995; 70: 337-41.
Sinico RA, Bottero P. Churg-Strauss angiitis. Best Pract Res Clin Rheumatol 2009; 23: 355-66.
Vaglio A, Casazza I, Grasselli C, Corradi D, Sinico RA, Buzio C. Churg-Strauss syndrome. Kidney Int 2009; 76: 1006-11.
Longmire M, Syed N, Allen RC. Churg-Strauss Syndrome with Orbital Inflammation. EyeRounds.org. September 27, 2010; Available from: http://www.EyeRounds.org/cases/119-Churg-Strauss-Syndrome.htm.

Ophthalmic Atlas Images by EyeRounds.org, The University of Iowa are licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License.