Chief Complaint: Blurry vision.
History of Present Illness: A 42-year-old female was referred to the University of Iowa Hospitals and Clinics (UIHC) Cornea Service from an outside ophthalmologist for evaluation of a 2-month history of blurry vision, photophobia, and mild, aching pain in both eyes.
The patient originally presented to her local ophthalmologist with blurry vision and photophobia in the right eye only. At that time her visual acuity was noted to be 20/70 in the right eye and 20/30 in the left eye. The slit lamp examination was reportedly significant for corneal edema and a mild anterior chamber inflammatory reaction that was present only in the right eye. On the basis of a presumptive diagnosis of anterior uveitis with corneal endothelial decompensation, she was placed on topical prednisolone acetate 1.0% four times daily, along with atropine sulfate 1.0% once daily and 5% sodium chloride ointment at bedtime. After 2 months of treatment there had been no improvement in the right eye and the new-onset of similar subjective symptoms and clinical findings in the left eye. Following initiation of treatment in the left eye, no clinical improvement has occurred.
Past Ocular History: As above, otherwise unremarkable.
Medical History: Huntington's disease and anxiety
Systemic Medications: Amantadine (Symmetrel®) 100 mg TID, bupropion HCL (Wellbutrin XL®) QD, clonazepam (Klonopin®) 0.5 mg BID, multivitamin QD, acetaminophen 500mg/diphenhydramine citrate 38mg (Excedrin P.M.) PO QHS
Ocular Medications: All topical medications were discontinued just prior to the consultation at the UIHC Cornea Service
Family History: Huntington's disease
Social History: Smokes three cigarettes per day for the past year and 1 pack per day prior to that for many years. Denies alcohol or illicit drug use.
Review of Systems: All negative except for complaints associated with Huntington's disease and anxiety
External exam: Normal
Visual acuity (with correction):
Current glasses:
Pupils: 5 mm in the dark and 2 mm in the light; no relative afferent pupillary defect.
Intraocular pressure (Tonopen):
Motility: Full motility OU.
Visual fields: Full to confrontation OU.
Central corneal pachymetry:
Dilated fundus exam: Normal OU
|
|
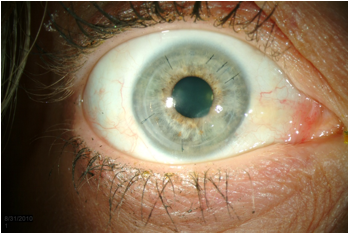
A tentative diagnosis of bilateral central corneal epithelial secondary to HSV disciform keratitis was made based on the previous history of anterior chamber inflammation and the localization of the edema to the central cornea. Although no changes consistent with Fuchs' endothelial dystrophy or other endothelial dystrophies were present in the peripheral cornea, the possibility of these conditions was entertained, along with the possibility of amantadine toxicity.
Treatment was initiated with topical prednisolone 1.0% six times daily. After 3 weeks of treatment the visual acuity had declined in the right eye to counting fingers at 3 feet and in the left eye to 20/250. This correlated with a bilateral increase in corneal edema. She was placed on systemic prednisone 40 mg daily and Valcyclovir (Valtrex®) 500 mg three times daily. Three weeks later there had been no change in the clinical course. The lack of clinical efficacy of topical and systemic steroids, along with the relatively short clinical course and absence of anterior chamber inflammation, suggested that a diagnosis of endothelial dysfunction secondary to severe or neglected HSV disciform keratitis was unlikely and that the clinical findings were more likely attributable to endothelial failure secondary to a heretofore unrecognized dystrophy or amantadine toxicity. The latter diagnosis was supported by specular microscopy which showed unidentifiable endothelial cells.
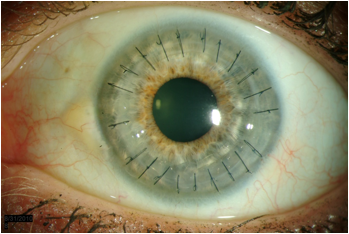
The patient underwent penetrating keratoplasty (PKP) in the right eye followed by the left eye several weeks later. She tolerated both procedures well. Both corneas were submitted to the Ocular Pathology Laboratory, and microscopic examination showed complete absence of endothelial cell without any guttae or stromal inflammation.
The post-operative course was uncomplicated. Six months after the PKP in the right eye and 3 months after PKP in the left eye, the best corrected visual acuity was 20/70 in the right eye and 20/60 in the left eye.
 |
 |
The postoperative corneal endothelial cell counts are currently being monitored carefully by specular microscopy to detect evidence of accelerated endothelial cell loss due to amantadine. In addition, her internist is considering the option of switching to a different medication to control the movement disorder associated with Huntington's disease.
Final Diagnosis: Bilateral corneal edema secondary to amantadine use
Amantadine is an organic compound originally used as an anti-viral for influenza A and also used as an anti-parkinsonian medication as it reduces extra-pyramidal symptoms and akathesia. The mechanism of action is poorly understood(PDR 2003).
Amantadine has been associated with a number of central nervous system side effects related to its dopaminergic, adrenergic, and anti-cholinergic properties. Ocular side effects include visual loss, mydriasis, oculogyric crises, punctate subepithelial opacities, and superficial punctate keratitis (Fraunfelder 1990, Fraunfelder 2001) In 1990, Blanchard published the first case of corneal edema associated with the use of amantadine. In 2004, Hughes et al. reported a case of 14-year-old boy taking amantadine who developed severe bilateral corneal edema in the absence of ocular inflammation. After removing the drug the patient had a quick and full visual recovery (Hughes 2004). In 2007, Kubo et al. described a similar case in which a 61-year-old Japanese male had complete reversal of bilateral corneal edema, with return of normal visual acuity, only 8 days after stopping the medication.
In 2007, a Veterans Health Administration survey was done to determine the correlation between corneal edema and the use of amantadine (French et al 2007). The group found a relative risk of corneal edema in patients taking amantadine of 1.7 (95% confidence interval: 1.1-2.8). In 2008, Jeng et al. presented a case series of three patients at the Cole Eye Institute with amantadine associated corneal edema. This report provided interesting results as the patients had been taking amantadine from 3 months to 6 years before the onset of symptoms, with resolution occurring in 2 of the patients. Histopathology on the keratoplasty specimen of the non-resolved case showed significant loss of endothelial cells with no guttae or evidence of stromal inflammation.
Since 2008, there have been a number of case reports of endothelial damage associated with amantadine (Chang et al. 2008, Pond et al. 2009, Esquenazi 2009, Koenig 2008, Chang et al. 2010), including a patient with graft failure after DSAEK (Koenig 2008). Most recently, Dr. Chang and associates at the Seoul National University College of Medicine performed a cross-sectional study on 169 patients taking amantadine for Parkinson's disease compared to age and gender matched controls with Parkinson's disease to evaluate the effect of amantadine on corneal endothelial cells in subjects with Parkinson's disease (Changet al. 2010). They found that the group taking amantadine had lower endothelial cell counts and lower hexagonality of endothelial cells. In addition, they found that longer duration of amantadine therapy and higher cumulative doses were associated with lower endothelial cell counts.
While the exact mechanism of endothelial damage from amantadine is unknown, it appears to be a well-documented side effect that is easily overlooked in the differential of corneal edema. Given the dose-effect relationship demonstrated recently and the potential for edema resolution after discontinuation of the drug, it is important to consider amantadine as a cause of corneal edema in all patients taking this medication.
In the present case, the complete loss of endothelium in both eyes on histopathological examination suggests that the condition had progressed beyond reversibility. Our current priority is to monitor careful for the earliest evidence of progression of endothelial cell density loss or morphologic changes at which time we hope to switch to an alternate medication for our patient's Huntington disease-related movement disorder.
Physician's Desk Reference (57th ed). Amantadine. Thompson Healthcare, Montvale, NJ; 2003:1213-1215.
Fraunfelder FT, Meyer SM. Amantadine and corneal deposits. Am J Ophthalmol 1990; 110:96-97.
Fraunfelder FT, Fraunfelder FW. Drug-Induced Ocular Side Effects (5th ed.), Butterworth-Heinemann, Boston; 2001:421-422.
Blanchard DL. Amantadine caused corneal edema [letter]. Cornea 1990;9:181.
Hughes B, Feiz V, Flynn SB, Brodsky MC. Reversible amantadine-induced corneal edema in an adolescent. Cornea 2004;23:823-824.
Kubo SI, Iwatake A, Ebihara N, et al. Visual impairment in Parkinson's disease treated with amantadine: case report and review of the literature. Parkinsonism Relat Disord 2007;14:166-169.
French DD, Margo CE. Postmarketing surveillance of corneal edema, Fuchs dystrophy, and amantadine use in the Veterans Health Administration. Cornea 2007;26:1087-1089.
Jeng BH, Galor A, Lee MS, et al. Amantadine-associated corneal edema potentially irreversible even after cessation of the medication. Ophthalmology 2008;115:1540-1544.
Chang KC, Kim MK, Wee WR, Lee JH. Corneal endothelial dysfunction associated with amantadine toxicity. Cornea 2008;27:1182-1185.
Pond A, Lee MS, Hardten DR, Harrison AR, Krachmer JH. Toxic corneal oedema associated with amantadine use. Br J Ophthalmol 2009;93:281.
Esquenazi S. Bilateral reversible corneal edema associated with amantadine use. J Ocul Pharmacol Ther 2009;25:567-570.
Chang KC, Jeong JH, Kim MK, et al. The effect of amantadine on corneal endothelium in subjects with Parkinson's disease. Ophthalmology 2010;117:1214-1219.
Koenig SB, McDermott ML, Simons KB. Nonimmunologic graft failure after Descemet's stripping automated endothelial keratoplasty (DSAEK) for presumed amantadine-induced corneal edema. Eye Contact Lens 2009;35:209-211.
Welder J, Kardon RH, Cohen A, Wagoner MD, Bilateral Corneal Edema Associated with Amantadine Use. Eyerounds.org. September 27, 2010; Available from: http://www.EyeRounds.org/cases/123-amantadine-corneal-edema.htm.

Ophthalmic Atlas Images by EyeRounds.org, The University of Iowa are licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License.