Chief Complaint: Decreased vision
History of Present Illness: A 44-year-old male presented to the University of Iowa Hospitals and Clinics for progressive decrease in vision in both eyes (OU) with "tremendous" glare symptoms.
Past Ocular History: Laser-assisted in situ keratomileusis (LASIK) OU in 2002 (8 years prior to presentation)
Past Medical History: Non-contributory
Medications: Clozapine, Ibuprofen, lithium, multivitamin
Allergies: None
Family History: Brother has similar corneal problems without LASIK. Mother had cataracts.
Social History: No alcohol use but smokes tobacco
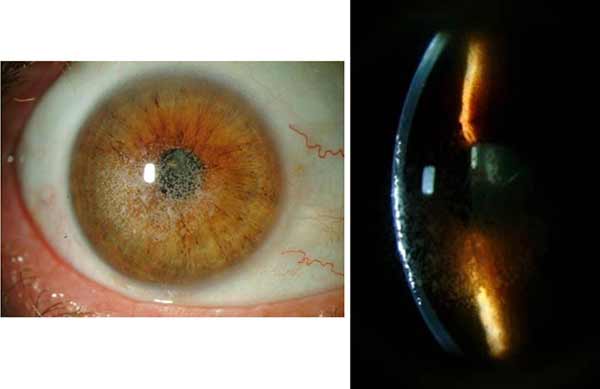 |
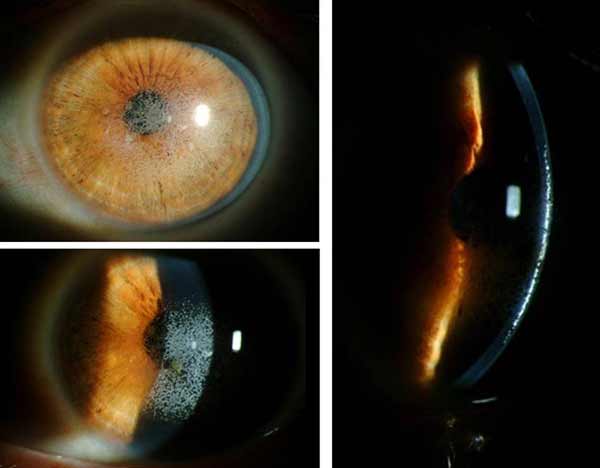 |
Clinical Course: Following the initial visit, the patient underwent a superficial keratectomy followed by phototherapeutic keratectomy (PTK) OU without complications. A slit lamp exam on the first post-operative day showed a reduction in the density of granular deposits in the visual axis. On post-operative day 7 the patient's vision remained poor at all distances, with visual acuity of 20/80 -1 OU. Due to the patient's poor vision, Intralase-enabled superficial anterior lamellar keratoplasty (IE-SALK) was recommended for both eyes, starting with the right eye. Slit-lamp photos of both eyes were obtained prior to surgery and are shown in figures 3 and 4.
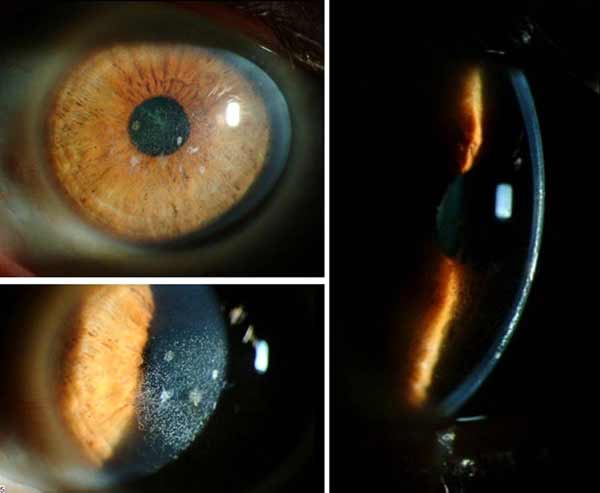 |
On post-operative day 3 after IE-SALK of the right eye, vision had not improved OD. However, although IE-SALK was not performed on the fellow eye, visual acuity had improved to 20/40 without correction OS since the superficial keratectomy and PTK performed over a month ago.(see figures 5 and 6 for photos of OD and OS, respectively).
 |
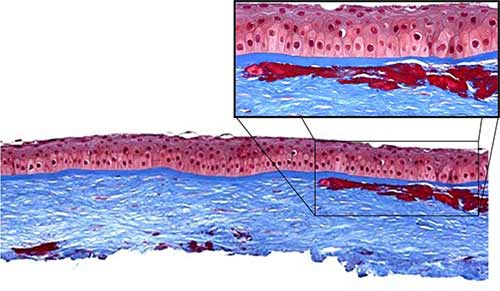 |
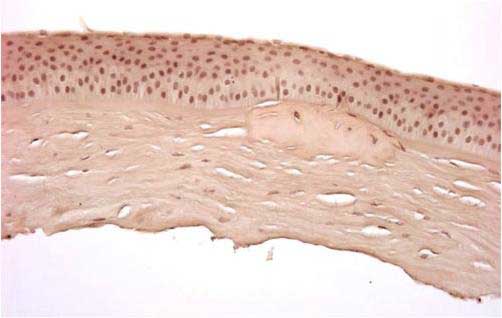 |
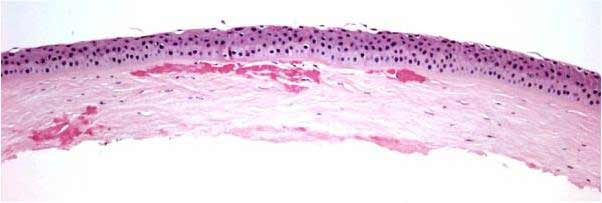
In general, all corneal dystrophies are classified by the anatomical layer of the cornea that is involved. This has been the case even after re-classification of the dystrophies in recent years (Weiss 2008, Birkholz 2009; please see the excellent review by Birkholz et al. for a more detailed review of corneal dystrophies: http://www.eyerounds.org/cases/43-Corneal-Stromal-Dystrophies.htm). The corneal stroma makes up roughly 90% of the total corneal thickness and is composed primarily of extracellular matrix (ECM) and stacked collagen fibrils. Keratocytes synthesize these stromal constituents and account for approximately 3-5% of the stromal volume. Keratocytes and their synthesized products are essential in maintaining the optical clarity of the cornea.
In 2008, the International Committee for Classification of Corneal Dystrophies (IC3D) was created by the Cornea Society for the purpose of publishing an updated classification scheme incorporating the recent advances in the genetics of corneal dystrophies (Weiss 2008, Poulaki 2008, Stone 1994). This new scheme still reflects the anatomic area affected (phenotype) but groups dystrophies according to genotypes involved. The corneal stromal dystrophies can be broken into two categories in the new scheme: 1) those having a mutation in the transforming growth factor, β-induced gene (TGFβI) and 2) those that are not known to result from a mutation in TGFβI gene. TGFBI protein was initially known as βIG-H3 (beta ig-h3).
*Lattice corneal dystrophy, gelsolin type (LCD2) has been reclassified as a systemic amyloidosis and is no longer classified as a corneal dystrophy by the new IC3D scheme
**Granular corneal dystrophy type 3, commonly known as Reis-Bücklers, is categorized in the stromal dystrophies due to its TGFβI genotype, although anatomically it is still classified as a Bowman's layer dystrophy.
*It is important to point out that although the three most common corneal dystrophies have classically been lattice, granular, and macular corneal dystrophies, the macular-type does not have the TGFβI genotype. Also, in relation to the other two, macular corneal dystrophy is inherited autosomal recessively and is the only one that affects the peripheral cornea, whereas lattice and granular corneal dystrophies are dominantly inherited and tend to affect the central cornea.
Table 1 highlights the epidemiology and genetics of the TGFβI corneal dystrophies.
| LCD Type 1 | CGD Type 1 | GCD Type 2 | |
|---|---|---|---|
| Age of Onset | First decade of life |
Early adolescence |
Variable, second decade of life |
| Laterality | Bilateral |
Bilateral |
Bilateral, one side affected more, early |
| Genetics | Autosomal Dominant |
Autosomal Dominant |
Autosomal Dominant |
| Chromosome of Gene(s) involved | 5q31 |
5q31 |
5q31 |
| Gene involved | TGFβI |
TGFβI |
TGFβI |
| Mutation noted in literature | R124C (exon 4) |
R555W (exon 12) |
R124H (exon 4) |
*Of historical note, the name Avellino was originally given to this corneal dystrophy because the first patients identified with the disease had ancestry tracing back to the Avellino region of Italy (Folberg 1988, Holland 1992).
Dystrophy |
Deposited Material |
Masson trichrome |
Alcian Blue |
PAS |
Congo Red |
Birefringence |
Macular |
Mucopolycaccharide |
- |
+ |
- |
- |
- |
Granular |
Hyaline |
+ |
- |
- |
- |
- |
Lattice |
Amyloid |
+ |
- |
+ |
+ |
* |
Granular Corneal Dystrophy Type 2 |
Hyaline/Amyloid |
+/- |
- |
+ |
+/- |
+/- |
Recent case studies have indicated that exacerbation or onset of symptoms in those with GCD2 can occur with LASIK surgery. The first case report by Wan et al. was important for two reasons: 1) the patient reported was of Korean descent, not Italian and 2) it showed that LASIK treatment appeared to exacerbate and accelerate findings of GCD2 (Wan 2002). Although articles published in 2000 and 2001 had reported that people of Japanese and Korean descent had high rates of GCD2 by genetic studies (72% and 90% respectively), the Avellino name was still commonly used in cornea-related publications (Mashima 2000, Kim 2001). In contrast, it has been stated that Europe and the US have proportionately higher rates of GCD1 and LCD compared to GCD2 (Mashima 2000). Therefore, special care may be warranted in patients of Asian descent prior to LASIK treatment in the case that they may have GCD2 (Lee 2003).
With regard to the natural history of heterozygous GCD2 (with no history of LASIK), it has been described that in many cases surgical intervention is not necessary until the sixth or seventh decade of life (Inoue 2002). However, additional case studies demonstrate that people with GCD2 are having accelerated disease following LASIK, LASEK and PRK within one to nine years post-treatment (Weiss 2008, Roo 2004, Aldave 2007, Lee 2008, Kim 2008). The peak age for LASIK and LASEK is between the third and fourth decades of life, suggesting that some patients will manifest symptoms of GCD2 at a much earlier age if they undergo refractive surgery and that these patients will require further surgical intervention to maintain good visual function.
While many possible explanations have recently been proposed for exacerbations after LASIK, the pathophysiology is not currently well-understood (Wan 2002, Aldave 2007, Kim 2008, Byoung 2010). Based on the findings that deposits are typically found at the interface of LASIK flaps, it has been suggested that damage to the corneal epithelium may exacerbate GCD2 findings after surgery (Roo 2004, Lee 2008, Chiu 2007). It is proposed that damage may be related to the secretion of TGF-β from damaged epithelial cells as part of normal wound repair. TGF-β secretion, however, stimulates mutated TGFβI protein product synthesis (kerato-epithelin or TGFβIp) resulting in a cascade of misfolding that results in accumulation of insoluble protein aggregates and clinical defect (Munier 1997).
Given that inflammation may play a role in GCD2 exacerbation after surgery, mitomycin C (MMC) has been studied as a way to mitigate this effect (Byoung 2010). However, a controlled trial did not show a statistically significant benefit from MMC used during refractive surgery in these patients (Byoung 2010). At present, limiting treatment depth at the time of refractive procedures appears to be the best way to prolong the need for penetrating keratoplasty in these patients (Kim 2010).
1988 |
First report of corneal dystrophy with characteristics of granular and lattice in people with linkage to province of Avellino, Italy |
1992 |
Further characterization of "Avellino Corneal Dystrophy", clinical manifestations and natural history |
1994 |
Genetic characterization of this dystrophy maps it to chromosome 5,long arm q |
1997 |
Further genetic studies isolate mutations for various TGFβI-related corneal dystrophies |
2000 |
Genetic studies show ACD (Rl24H mutation) present in Japanese patients with corneal dystrophy (Avellino name no longer favored) |
2001 |
Genetic studies into Korean patients demonstrate R124H mutations, and that these account for 90% of anterior corneal dystrophy |
2002 |
First case report of Korean female with exacerbation of GCD2 after LASIK |
2004 |
Continued studies showing more individuals with exacerbation of GCD2 after LASIK (n=7, Korean patients) |
Davis AS, Syed NA. Granular Corneal Dystrophy Discovered Following LASIK: A Clinicopathologic Correlation of Granular Corneal Dystrophy Type II and LASIK. EyeRounds.org.September 16, 2011. Available from: https://eyerounds.org/cases/140-post-LASIK-Granular-Corneal-Dystrophy.htm.

Ophthalmic Atlas Images by EyeRounds.org, The University of Iowa are licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License.