Chief Complaint: 38-year-old female with persisting tearing for 6 months.
History of Present Illness: This young patient has a known history of T3N2M0 breast cancer. After biopsy-proven diagnosis with infiltrating ductal carcinoma, the patient underwent weekly neoadjuvant therapy with carboplatin, trastuzumab (Herceptin®), and docetaxel (Taxotere®). With further nodules thereafter being biopsy-positive for infiltrating ductal carcinoma of the breast, the patient later underwent radical mastectomy with lymph node dissection and subsequent post-operative radiation therapy.
It was during the first two months of neoadjuvant therapy that the patient first began to notice "watery eyes". Within weeks, her teary eyes progessed to true epiphora that became bothersome. It was during this time, however, that the patient was dealing with the further progression and treatment of her primary disease. As such, she was not referred to ophthalmology for evaluation until several months after completion of therapy for her breast cancer.
Complaints now are of persisting epiphora, both eyes (see Figure 1). The increased tear film causes mild blurring to her vision. There is no other ocular complaint.
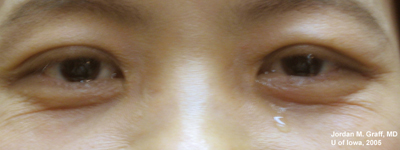 |
Medical and Ocular History: T3N2M0 infiltrating ductal carcinoma, as noted. Otherwise unremarkable.
Social History: Patient lives near Iowa City. She does not drink alcohol nor smoke.
Family History: Noncontributory
Medications/Allergies: Acetaminophen, Lorazepam, Ondansetron, and Trazodone—all PRN. Patient has NKDA.
ROS: Some localized tenderness and tightness over the chest wall at the site of mastectomy and radiation therapy. Otherwise negative, except as noted above
Ocular Exam:
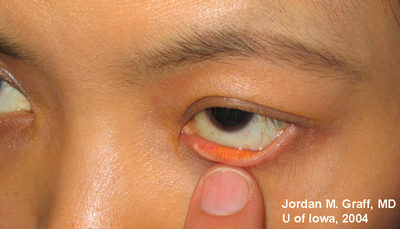 |
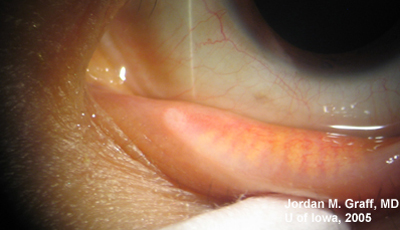 |
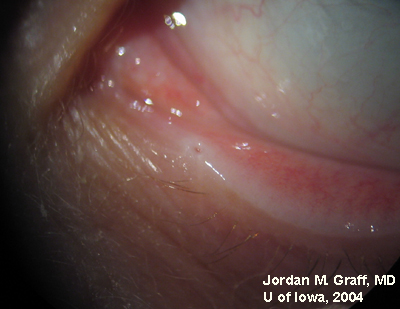 |
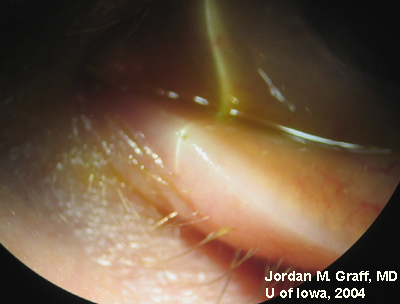 |
Punctal dilation was attempted using a fine tapered punctal dilator with minimal success. Thereafter, a small pediatric lacrimal probe (0.50mm) was advanced through all four punta and a hard stop was acheived each time. Thus, while we could determine that the lacrimal drainage system was not completely closed off, significant stenosis was clearly evident with resultant epiphora.
Docetaxel (Taxotere®) is one of a family of taxoid chemotherapeutic agents. Like its older cousin, Paclitaxel (Taxol®), Docetaxel is extracted from the bark or needles of the yew tree. The taxoid drugs work against cancer by interfering with the process of mitosis. Docetaxel acts by binding to microtubules and preventing their disassembly; thus, by freezing the cell in mitosis, cell division is unable to take place and the cell will eventually die.
Epiphora as a side effect from Docetaxel therapy was first described in Ophthalmology in 2001 (1). Since then, several related reports have surfaced. Up to 50% of patients on weekly Docetaxel therapy have reported symptoms of epiphora (2), and the symptoms usually do not resolve upon completion of antineoplastic therapy. Examination of these patients reveals significant punctal, and sometimes canalicular, stenosis.
It is hypothesized that the drug is secreted in the tear film and that a direct effect from the chemotherapeutic agent on the mucosa results in fibrosis of the punctum and canalicular system (4). Alternatively, since some fibrosis has been reported elsewhere in the body of patients on docetaxel (3), the mucosal fibrosis in the lacrimal system may be secondary to the systemic effects of the drug. Of note, similar symptoms of dacryostenosis had been reported many years ago with the chemotherapeutic agent 5-fluorouracil (5, 6). (There is no report in the literature of similar effects from either of the other agents our patient was taking, carboplatin and trastuzumab).
Review of this problem with physician colleagues in oncology has led to some recommendations regarding prevention or treatment of punctal and canalicular stenosis (7). The treating oncologist may want to consider referral to an ophthalmologist for prophylactic bicanalicular silicone intubation early in the course of docetaxel therapy to prevent complete stenosis of the drainage system. Alternatively, early referral could be reserved for those patients who begin to have symptoms during the course of treatment. However, if near complete stenosis does occur, attempts at bicanalicular silicone tube placement may not be successful and the patient may require dacryocystorhinostomy (DCR) with pyrex tube insertion (Jones tube) for symptom relief. This, of course, would be less desirable. Prevention of stenosis would be preferred.
In summary, this patient suffers epiphora secondary to punctal and canalicular stenosis from docetaxel therapy. The patient declined offers for oculoplastic service evaluation and bicanalicular silicone intubation or lacrimal drainage reconstruction. For now she feels that her symptoms are bothersome, but not intolerable. She will reconsider these options if, at a later date, she feels that her symptoms are intolerable.
EPIDEMIOLOGY
|
SIGNS
|
SYMPTOMS
|
TREATMENT
|
Graff JM, Johnson AT. Punctal and Canalicular stenosis (dacryostenosis) from Docetaxel: 38-year-old female with persisting tearing for 6 months.. EyeRounds.org. February 21, 2005; Available from: http://www.EyeRounds.org/cases/31-dacryostenosis.htm

Ophthalmic Atlas Images by EyeRounds.org, The University of Iowa are licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License.