INITIAL PRESENTATION
Chief Complaint: 71-year-old man referred for evaluation of possible uveal lymphoma in left eye
History of Present Illness: A 71-year-old man was referred to the University of Iowa Retina and Vitreous Clinic for evaluation for possible uveal lymphoma of the left eye. He was first seen locally for blurred vision in both eyes and was found to have subretinal fluid in the left eye. This was initially concerning for uveal effusion syndrome. Laboratory workup at that time with complete blood count, lyme titers, syphilis antibody testing, soluble IL-2 receptor, quantiferon gold, serum protein electrophoresis (SPEP), rheumatoid factor, and chest x-ray showed an elevated soluble IL-2 receptor but was otherwise unremarkable. He was subsequently lost to follow up for a period of about a year due to the COVID-19 pandemic. He then returned locally with worsening subretinal fluid and concern for a “salmon patch” lesion on the conjunctiva of the left eye.
On presentation to our clinic, the patient reported chronic blurred vision in the left eye without acute changes. He had no eye pain.
Past Ocular History:
Medical History:
Medications:
Allergies: Iodinated contrast media
Family History:
Social History: Non-contributory
Review of Systems: Negative except for what is detailed in the History of Present Illness
OCULAR EXAMINATION
OU: Normal, no proptosis
OD
OS
OD
OS
B-Scan Ocular Ultrasound
Ocular Coherence Tomography (OCT) Macula
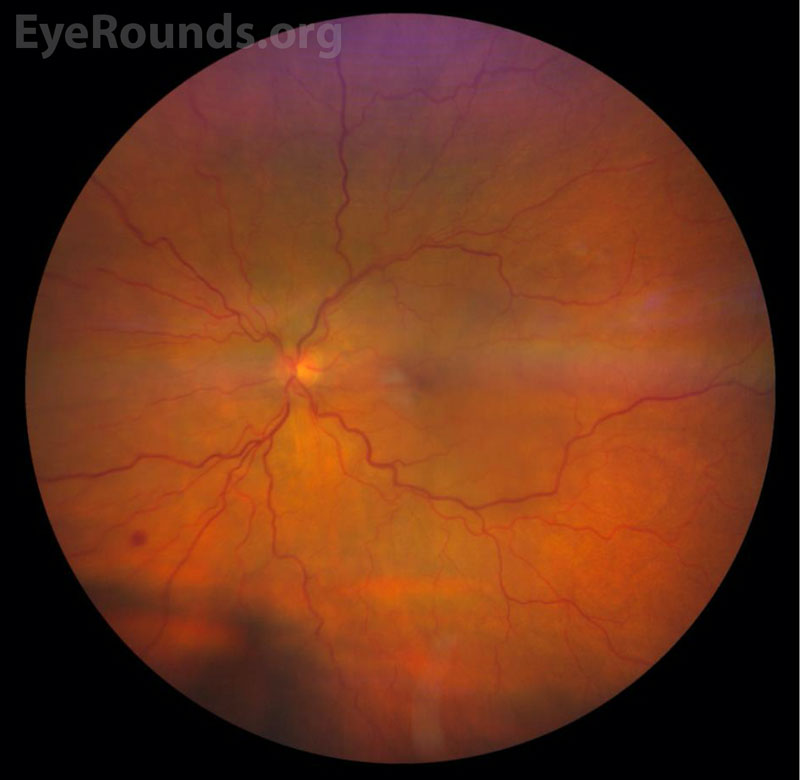
Color Fundus Photography
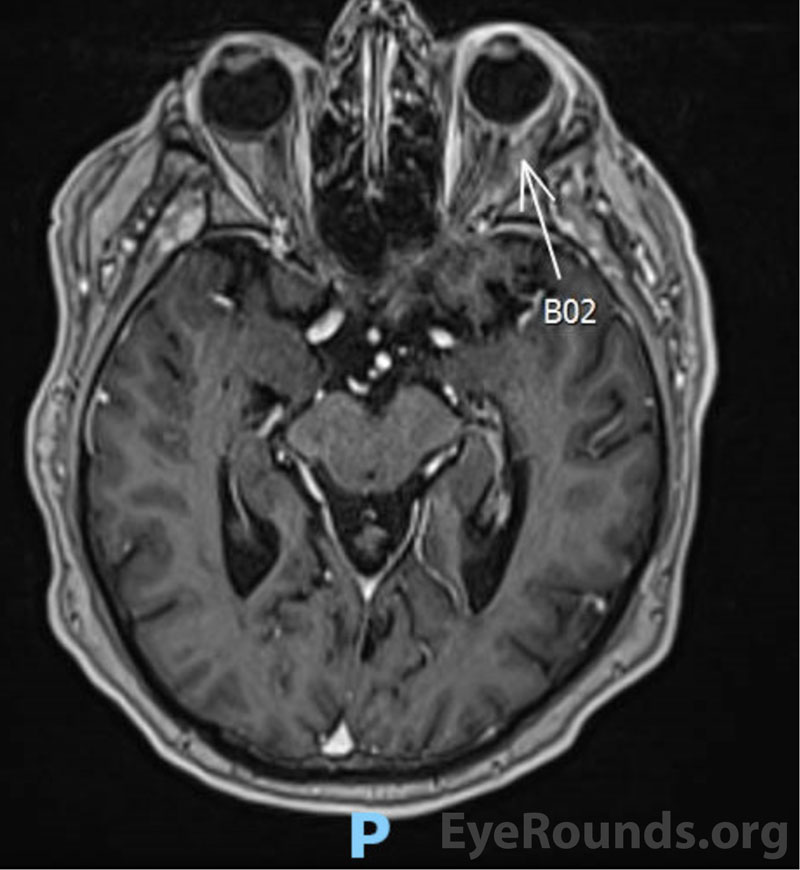
Magnetic Resonance Imaging (MRI)
DIAGNOSIS AND CLINICAL COURSE
The patient underwent biopsy of the subconjunctival, salmon-colored mass which returned a diagnosis of extranodal marginal zone B-cell lymphoma. The patient underwent CT scans of the chest, abdomen, and pelvis which showed no evidence of extra-ocular disease but incidentally identified a bladder mass which was ultimately confirmed to be bladder cancer. Bone marrow biopsy was negative for systemic disease. He was referred to radiation oncology and completed twelve fractions of external beam radiation to the left eye (24 Gy). The response to treatment was excellent with resolution of the subconjunctival infiltrate. The patient completed additional chemotherapy for bladder cancer in March 2021. He has had no evidence of recurrence.
Differential diagnosis:
DISCUSSION
Epidemiology
Intraocular lymphomas represent 1%-2% of extranodal lymphomas and 1.86% of all malignant ocular tumors [1]. Uveal lymphoma is the rarest form of intraocular lymphoma, with approximately 80 cases reported in the literature since 1920 [1,2]. Incidence demonstrates a slight predominance in males with no apparent racial predilection [1,3,4]. Uveal lymphomas are primarily non-Hodgkin’s lymphomas of B-cell origin and can present as a primary manifestation or secondary metastasis of systemic disease [2,5,6]. Primary choroidal lymphomas are the most common and are typically low-grade with indolent growth, although in rare cases systemic dissemination does occur [2,7]. Extranodal marginal zone lymphoma (EMZL) of B-cell origin, also known as mucosa-associated lymphoid tissue (MALT) lymphoma, is the most common form of primary uveal lymphoma and accounts for 60% to 80% of cases [2]. The mean age of diagnosis is between 50 and 60 years with an overall range of 15-85 years of age [1]. Primary uveal lymphoma can involve the choroid, ciliary body, or iris, although ciliary body and iris involvement are very rare [1,2,3,8]. Secondary uveal involvement from systemic lymphoma is rare, and the exact incidence is uncertain [7].
Presentation/Signs/Symptoms
Uveal lymphoma can be difficult to diagnose due to its rarity and its tendency to masquerade as other forms of ocular disease (see “Differential Diagnosis”) [2,9]. This “masquerade syndrome” contributes to delays in diagnosis and proper treatment following onset of symptoms [2,9]. The most common symptoms of early-stage uveal lymphoma are typically painless and include decreased vision and metamorphopsia secondary to exudative retinal detachment [2,7,9]. The most common signs include diffuse choroidal thickening, conjunctival infiltrate, and elevated intraocular pressure [2]. In primary uveal lymphoma, the vitreous remains clear and there is no central nervous system involvement [2,7]. Advanced primary or secondary disease may be painful and can cause severely reduced vision and total retinal detachment, diffuse thickening of the uveal tract, and proptosis if there is orbital involvement [2,7,9]. Infiltration of the optic nerve and meninges can occur, although this is rare and most commonly occurs in advanced stages of disease [7,8,9]. Fundus examination reveals one or multiple characteristic yellow-white choroidal infiltrates and choroidal folds [2]. Episcleral and conjunctival extensions can result in a characteristic salmon-colored patch easily visible upon external examination [2,10].
Pathophysiology/Etiology
The etiology of uveal MALT lymphomas, or EMZL, is unknown, but research suggests development secondary to antigenic stimulation by autoantigens or by microbial pathogens, which results in irregular accumulation of lymphoid tissue in sites of disease [1,5]. Although uveal lymphomas are most commonly primary lymphomas, secondary intraocular manifestation can occur from systemic disease [2]. There is a notable association between an increased risk of MALT lymphomas and several autoimmune diseases such as Sjogren’s, Hashimoto thyroiditis, and lymphoid interstitial pneumopathy [5,11]. Pathogenic infection with Helicobacter pylori is reported to have an association with secondary development of gastric MALT lymphoma [12]. Additionally, chronic infection with malaria protozoa, Borrelia burgdorferi, Campylobacter jejuni, and Chlamydia psittaci bacteria, and hepatitis C have shown possible implications in the development of MALT lymphomas, although discussion of evidence in the literature remains controversial [1,5,11-14].
Testing/Laboratory work-up/Imaging
Biopsy is usually indicated to confirm the diagnosis [2]. Staging, characterization of disease extension in the eye, and detection of systemic involvement is critical [2,15]. This typically requires a combination of biopsy, ancillary imaging, histology, immunohistochemistry, and molecular analysis [2,15]. B-cell EMZL shares many histopathologic characteristics with other MALT-type lymphomas, such as lymphoid hyperplasia. Flow cytometry is useful for classifying the immunophenotype and subtype of lymphoma to provide further diagnostic accuracy [16].
Evaluation of disease extent in the orbit can be evaluated with CT, MRI, and B-scan ultrasonography [2]. Imaging is helpful for assessing choroidal thickening, extra-scleral extension, and involvement of adnexal structures [2]. Fluorescein angiography and indocyanine green angiography (ICG) can be used to image the choroidal infiltrates as well [2,17]. Compared to fluorescein, ICG utilizes higher-wavelength absorption and emission spectra. ICG also has a greater ability to bind proteins in serum, which limits its diffusion out of the choriocapillaris. These properties typically allow ICG to provide higher-resolution imaging of choroidal lesions [17,18]. Areas of hypocyanescence are frequently present and may correspond to lymphoma cells blocking perfusion of choroidal vessels [2,17].
Evaluation for systemic involvement and staging includes CT and MRI of the neck, chest, abdomen, and pelvis, full-body PET scan, complete blood count, complete metabolic panel, and bone marrow biopsy [2,19], often performed in conjunction with a medical oncologist.
Treatment/Management/Guidelines
Uveal lymphomas of the extranodal marginal zone B-cell type are most frequently low-grade [2]. In cases of local orbital disease, prognosis is usually favorable, and complete remission is often achieved following treatment [2]. The prognosis for cases with systemic involvement is also good, with treatment often resulting in complete remission, partial remission, or asymptomatic, stable disease [1,2,10].
Due to its rare occurrence, no standardized treatment guidelines for uveal lymphoma have been established [2,5]. Treatment of localized uveal lymphoma with external beam radiation therapy (EBRT) is the most frequently reported treatment leading to full remission in many cases [1,2,16,20]. EBRT dosage corresponds to lymphoma grade, and the total dose can range from 28 to 40 Gy [1,2]. Side effects from EBRT can include cataract formation, dry eye syndrome, neovascular glaucoma, radiation retinopathy, and superficial keratopathy [21]. Treatment with rituximab (anti-CD20 monoclonal antibody) or chemotherapy tends to be reserved for cases with systemic involvement [2,5]. Side effects from rituximab are typically less severe than those from EBRT, with the most common being fever, chills, throat irritation, and temporary B-cell depletion [21]. A retrospective clinical review described the treatment outcomes for 21 patients diagnosed with primary uveal lymphoma and one patient with a history of follicular lymphoma plus uveal involvement [2]. Localized, monocular involvement was observed in 10 patients, binocular involvement was observed in 12 patients, and extraocular systemic disease was observed in 5 patients [2]. All patients with localized monocular disease who received EBRT achieved 100% remission with a median follow-up of 28.7 months [2]. In the remaining patients who were treated for binocular or systemic disease with EBRT, rituximab, or chemotherapy, 78.6% achieved complete remission with a median follow-up of 30.3 months [2]. Among the patients treated with rituximab only, 1 patient achieved a stable, asymptomatic disease state and 2 patients achieved partial remission [2]. No patients developed new systemic disease after the onset of treatment through the median follow-up of 30.3 months, and no patients passed away from lymphoma-related complications [2].
EPIDEMIOLOGY AND ETIOLOGY
|
SIGNS
|
SYMPTOMS
|
TREATMENT/MANAGEMENT
|
References
Hartness EM, Shah TJ, Hendricks TM, Binkley EM. Uveal Lymphoma. EyeRounds.org. January 9, 2023. Available from https://EyeRounds.org/cases/335-uveal-lymphoma.htm

Ophthalmic Atlas Images by EyeRounds.org, The University of Iowa are licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License.