
The University of Iowa
Department of Ophthalmology and Visual Sciences
August 22, 2013
Glaucoma is the second most common cause of blindness worldwide, and is the leading cause of irreversible blindness in the world. Primary open angle glaucoma (POAG) is the most prevalent type of glaucoma in the United States and affects about 2% of individuals over age 40 (1, 2). Blindness due to glaucoma in the US is estimated to cost over $1.5 billion annually, and the problem is expected to increase as the population ages [3].
Glaucoma is a chronic, progressive optic neuropathy characterized by thinning of the neuroretinal rim of the optic disc [4]. It results in a characteristic appearance of the optic nerve head called cupping, and a corresponding loss of visual field. Importantly, the early symptoms of glaucoma can be quite subtle, and over half of individuals affected are not aware that they have the disease. Early recognition is important because the prognosis can be good if patients are diagnosed and treated appropriately early on in the disease process, but if left untreated it can progress to irreversible blindness [2]. The major risk factors for glaucoma include advanced age, family history, African race, thin corneas, and high intraocular pressure (IOP). The only risk factor that is treatable is high IOP, and all current therapies for POAG are aimed at decreasing IOP [3, 5].
Intraocular pressure is determined by the balance between the inflow and outflow of aqueous humor in the anterior chamber of the eye. Aqueous humor is produced by the ciliary body and is secreted posterior to the iris. It then flows through the pupil and circulates throughout the anterior chamber to supply nutrients to the lens, iris, and cornea. The majority of the aqueous humor then drains through the trabecular meshwork which lies in the iridocorneal angle. It then enters the canal of Schlemm and eventually reaches the venous circulation. A small amount of the aqueous humor also exits the anterior chamber through the ciliary body face and iris root in a mechanism called uveoscleral outflow. In contrast to angle closure glaucoma where the iris lies over the trabecular meshwork and blocks the outflow of aqueous humor, the iridocorneal angle is open in POAG and the resistance to outflow is within the trabecular meshwork itself [3, 4].
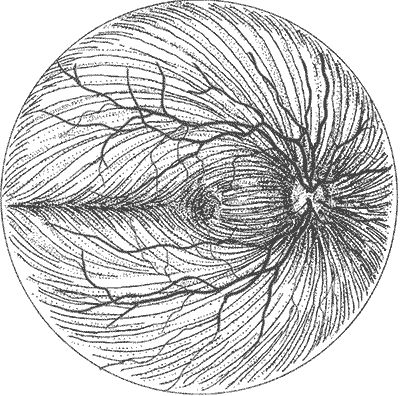
The optic nerve is composed of axons of retinal ganglion cells, which transmit visual information from the retina to the brain. The optic disc is the portion of the optic nerve visible in the posterior segment of the eye. The fibers from the nasal side of the retina travel directly to the optic disc while the fibers from the temporal side follow a more complex pattern. Those from the macula travel straight to the optic disc via the papillomacular bundle, and those from the periphery form the superotemporal and inferotemporal arcs around the papillomacular bundle. The axons on the temporal side do not cross the horizontal midline [2, 3].
In open angle glaucoma, the iridocorneal angle is open, meaning that the iris is not overlying the trabecular meshwork and preventing aqueous outflow. Open angle glaucoma can be further categorized as either primary or secondary depending on whether there is an identifiable underlying cause for the glaucoma such as medication side effects or excess pigmentation within the eye.
It is important to recognize that elevated IOP is simply a risk factor for glaucoma and not part of the definition of glaucoma. Glaucoma can be present without elevated IOP. If the IOP is normal but the patient has optic nerve damage and visual field loss, it is called normal tension glaucoma.
Patients with increased IOP but no optic nerve damage or visual field loss are said to have ocular hypertension [2, 4].
Glaucoma is almost unique among optic neuropathies in that it results in cupping rather than pallor of the optic nerve head. It is believed to be the loss of the retinal nerve fiber layer (RNFL) tissue that leads to the increased cup to disc ratio [6]. The pathophysiology behind the loss of the RNFL in glaucoma is not fully understood. There is ongoing research into how high IOP and other factors lead to the nerve damage and cupping seen in glaucoma [3, 4].
POAG is generally asymptomatic until late in the course of the disease. The primary symptom is loss of visual field, but there is usually such a gradual decrease in peripheral vision that it may not be noticeable to the patient until the vision loss is advanced. Furthermore, studies have shown that up to half of the retinal ganglion cells may be lost before there are signs of visual field loss on exam [2, 3].
Clinical findings of POAG include an open iridocorneal angle, characteristic changes of the optic disc, and – if sufficient optic nerve damage has occurred - corresponding visual field loss. Elevated IOP is a common finding and is followed clinically because elevated IOP is the only modifiable risk factor in glaucoma. Studies have repeatedly demonstrated that decreasing IOP can slow both the onset and progression of glaucoma, even in patients who have never had an elevated IOP. All current treatments for POAG work by decreasing IOP [4].
Examination of the optic disc at the slit lamp or with indirect ophthalmoscopy is useful for diagnosing early glaucoma since optic disc changes occur before loss of visual field is noticeable. Direct ophthalmoscopy is somewhat more difficult due to the lack of a stereoscopic view but can still be used to look for characteristic changes [3]. The key findings on examination of the optic nerve head include:
It is also useful to examine the RNFL adjacent to the optic disc. The normal bright reflections from this layer are lost in glaucoma as the nerve fiber layer thins [3].
Gonioscopy allows for visualization of the iridocorneal angle, which cannot be seen directly through the cornea. This is important in distinguishing POAG from other causes of glaucoma such as angle closure glaucoma or secondary forms of open angle glaucoma [1]. For examples of gonioscopy, visit www.gonioscopy.org.
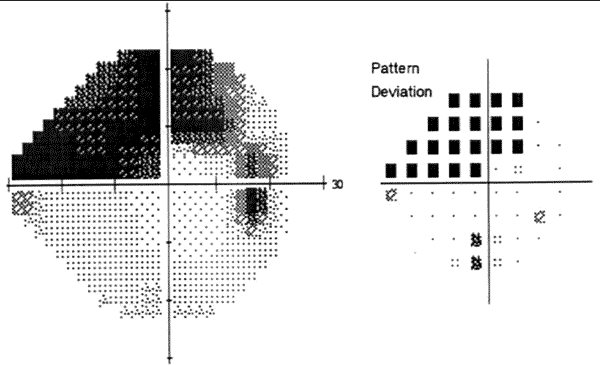
Perimetry is used to evaluate a patient’s visual field. It is useful both in the diagnosis of glaucoma and in following changes over time to determine whether the disease is progressing [2]. The most commonly used forms of perimetry employ a small white light against a dim white background to test the function of the retinal ganglion cells [7]. Visual field abnormalities associated with POAG include:
Patients often undergo repeated visual field testing to evaluate for new changes in their visual field in order to guide treatment [1].
OCT can measure the thickness the different portions of the retina, inducing the RNFL and the optic disc. Studies have shown that there is not always high correspondence between changes in the RNFL detected by OCT and visual field changes. However, it is believed that determining the rate of RNFL changes is clinically relevant and can help determine prognosis and treatment plans for glaucoma patients [3, 8, 9].
POAG would seem to be a good candidate for population wide screening because it is relatively common, has a long latent period, and most cases can be prevented or limited with currently available therapy. Population-based screening using IOP measurement, assessment of the optic disc and RNFL, and visual field testing has been studied extensively but is not currently considered cost-effective [1]. Some researchers have suggested that general practitioners can help identify POAG patients by using direct ophthalmoscopy and confrontational visual fields. However, this is not an ideal substitute for regular eye exams, especially in patients with a family history of glaucoma, advanced age, or those of African descent, all of whom are at higher risk of POAG [6].
The goals of treatment in POAG are to control IOP in a target range and to maintain stable optic nerves, RNFL, and visual fields. Studies have shown that the lower the IOP, the less likely there will be progression of glaucomatous damage. The target IOP is different for each patient and is the pressure at which it is thought that the patient will not sustain further damage. This is often estimated by reducing the pre-treatment pressure by 20-30%. The target may become even lower if the patient continues to have progression of glaucoma within their previous target range [1].
The major classes of treatment for POAG include medications, laser therapy, and surgery.
Topical medications are generally the initial intervention used to lower IOP. The commonly used drugs include:
These drugs are often used in combination in an attempt to reach the target IOP. Although these drugs are used topically, they are strong enough to cause systemic side effects. These should be assessed for in all patients taking topical medications for POAG [1].
Laser trabeculoplasty lowers IOP by increasing the outflow of aqueous humor through the trabecular meshwork; the exact mechanism is unknown. It can be performed with argon laser (ALT) or frequency-doubled YAG laser (selective laser trabeculoplasty). Trabeculoplasty may be considered as initial therapy in the place of medications, but it is more often used after patients have been on maximally tolerated medical therapy but remain above their target IOP [1, 2].
Incisional surgical therapy is typically performed when medical and laser therapies have provided inadequate IOP control.
Trabeculectomy is the most common surgery used to treat glaucoma. It works by creating an alternative outflow path for aqueous humor, which is essentially a fistula between the anterior chamber and the subconjunctival space [10]. Trabeculectomy alone has a failure rate of up to 30% after ten years, but this rate can be decreased by using antifibrotic medications such as mitomycin C during surgery to prevent scarring of the subconjunctival space [1].
Aqueous shunts, also called setons, are a small tube that inserts into the anterior chamber and drains aqueous humor to a plate sutured into place on the equatorial portion of the eye. The aqueous then drains into a fibrous capsule that forms around the plate and diffuses out. Shunts are usually considered when trabeculectomy has failed or is not expected to be successful, as may occur with several forms of secondary open angle glaucoma [1].
There are several other minimally invasive procedures that are being used to treat POAG. These include canaloplasty and trabectome. The indications for and success rates of these procedures remain controversial at this time [1].
Laser cytophotocoagulation decreases the rate of aqueous production by destroying some of the ciliary body. Because there is some potential for degradation of central acuity, it is typically used only in glaucoma that is refractory to all other therapies and in eyes with poor visual potential [1].
Petersen C, Alward WLM. Primary Open Angle Glaucoma: From One Medical Student to Another: From One Medical Student to Another. EyeRounds.org. August 21, 2013; available from https://eyerounds.org/tutorials/POAG/