


The University of Iowa
Department of Ophthalmology and Visual Sciences
Posted May 3, 2024
Idiopathic intracranial hypertension (IIH), previously known as pseudotumor cerebri and benign intracranial hypertension, is an increasingly common cause of visual field damage across the United States. As its name suggests, IIH is characterized by increased intracranial pressure with symptoms that can mimic those seen by mass lesions within the cranial vault (hence the former name pseudotumor cerebri).
Between 1990 and 2001, the overall incidence of IIH in the United States was approximately 1/100,000. (1) However, from 2002 to 2014, its incidence has more than doubled to 2.4/100,000. (1) This increase has been associated with a concurrent rise in the average population body mass index (BMI) and the ongoing obesity epidemic. For example, the incidence of IIH among women with a BMI between 18.5-24.9 is 6.8/100,000. However, for women with a BMI ≥ 25, this increases to 22/100,00. (1) Notably, the vast majority of IIH patients are female. When strict criteria are applied, approximately 97.5% of patients are women. (2) Therefore, the most significant risk factors for the development of IIH are obesity, recent weight gain, and female sex. (3) Due to its strong association with obesity, the incidence of IIH is heavily affected by the population being studied and the demographics in that region. The classic patient with IIH is a female between the ages of 20-50 who are overweight or have had a recent increase in weight. IIH rarely affects men, and alternative diagnoses or secondary causes of increased intracranial pressure (ICP) should be strongly considered and worked up in men with possible papilledema.
IIH can cause significant impairment in a patient’s quality of life, and its most significant complication is visual field loss secondary to elevated intracranial pressure. Vision loss is often partially to fully reversible, and several treatments can reduce intracranial pressures and halt disease progression. While multiple distinct causes can lead to increased intracranial pressure, IIH is unique in that no specific cause can be identified on history and examination.
The cranial vault is composed of three major components: the brain, blood, and cerebrospinal fluid (CSF). Since these components are constrained within the fixed volume of the skull, an increase in any one of these three components would lead to elevated intracranial pressures. IIH is characterized by an increase in intracranial pressure secondary to increased CSF pressure (also often referred to as increased ICP).
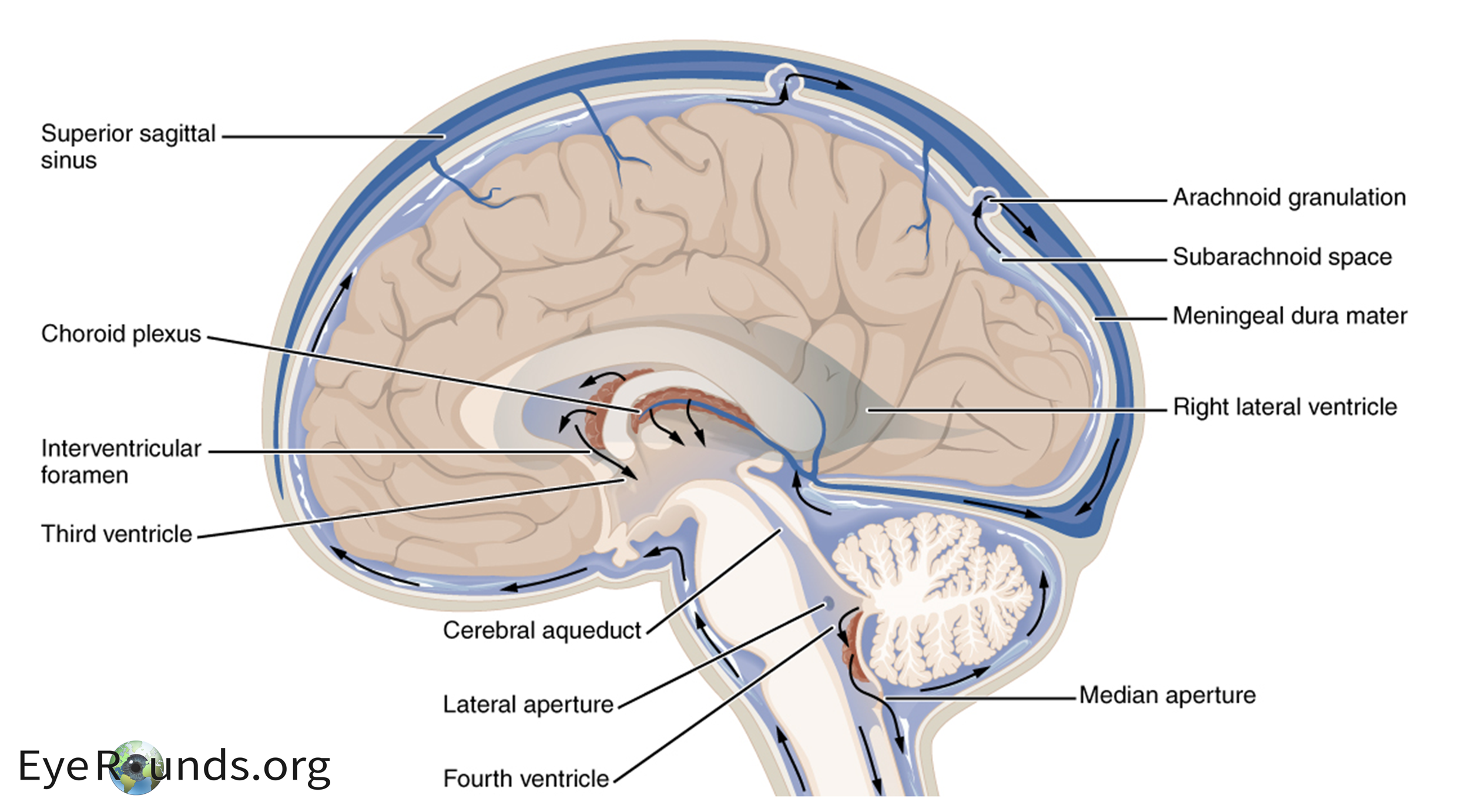
The majority of CSF is produced by the choroid plexus within the lateral ventricles of the brain. An important enzyme in the production of CSF is carbonic anhydrase, which catalyzes the conversion of CO2 and H2O to HCO3- and H+. As CSF is secreted from the lateral ventricles, it traverses through the ventricular system and subarachnoid space. Eventually, it is resorbed at the arachnoid granulations which drain to the venous system via the superior sagittal sinus (Figure 1 (4)). The exact cause of this increased CSF pressure is unknown. However, impaired or blocked absorption at the arachnoid granulations is most likely. (5) Other diseases that result in intracranial pressure can mimic IIH including venous sinus thrombosis, vitamin A toxicity, danazol, tetracyclines, and excessive growth hormone. Anatomic secondary causes for increased ICP should also be worked up (e.g. Chiari or intracranial tumors).
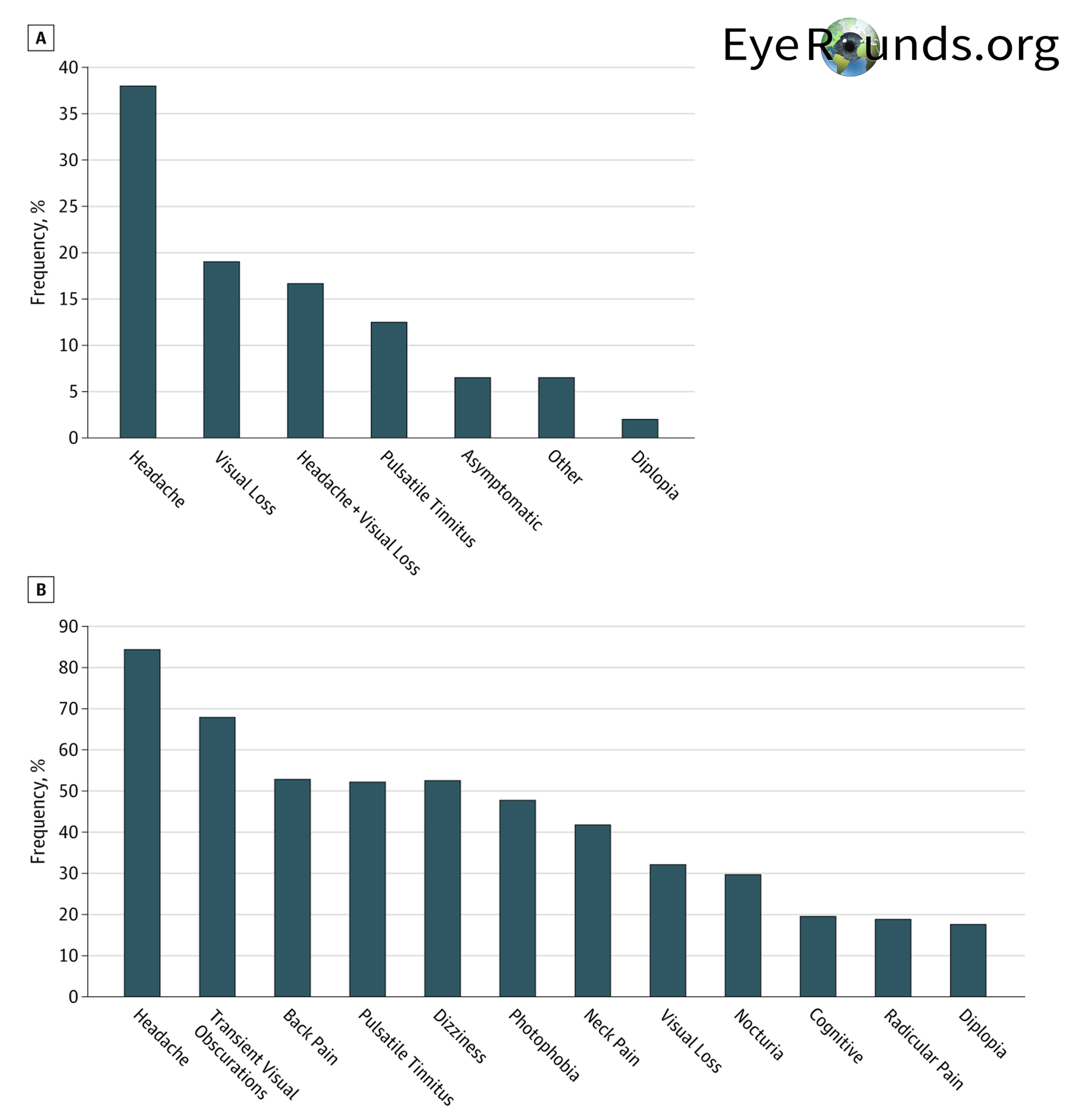
IIH typically has an insidious onset and is progressive. As reported by the Idiopathic Intracranial Hypertension Treatment Trial (IIHTT), the most frequently reported symptoms in patients include headache (84%), transient visual obscurations (68%), back pain (53%), and pulsative tinnitus (52%) which is demonstrated below in Figure 2. (2) Headache is the chief complaint for most patients. These headaches are typically severe, throbbing, occasionally localized behind the eye, and can be worsened with coughing or straining. Additionally, they are characteristically worse in the morning due to gravity-dependent CSF redistribution after lying flat and can likewise be improved by standing. Transient visual obscurations, the second most common symptom, are brief episodes (usually less than 30 seconds) of cloudy or black vision that can occur in one or both eyes. IIH can lead to back pain due to increased CSF pressures causing spinal nerve root compression (similar to papilledema). Other less common symptoms include dizziness, photophobia, neck pain, nocturia, cognitive changes, and diplopia. (2)
IIH is diagnosed with the Modified Dandy Criteria where all five criteria have to be met for a diagnosis of IIH to be made (6):
As the criteria above suggests, every patient with suspected IIH should be worked up with MRI (+/- MRV) along with lumbar puncture prior to solidifying the diagnosis of IIH (see below for more details on this).
A significant portion of the evaluation for IIH includes ruling out other causes of increased intracranial pressure, and a comprehensive ophthalmological and neurological exam is critical for this. In most patients, bilateral papilledema and its attendant visual loss are commonly the only findings (Figure 3).
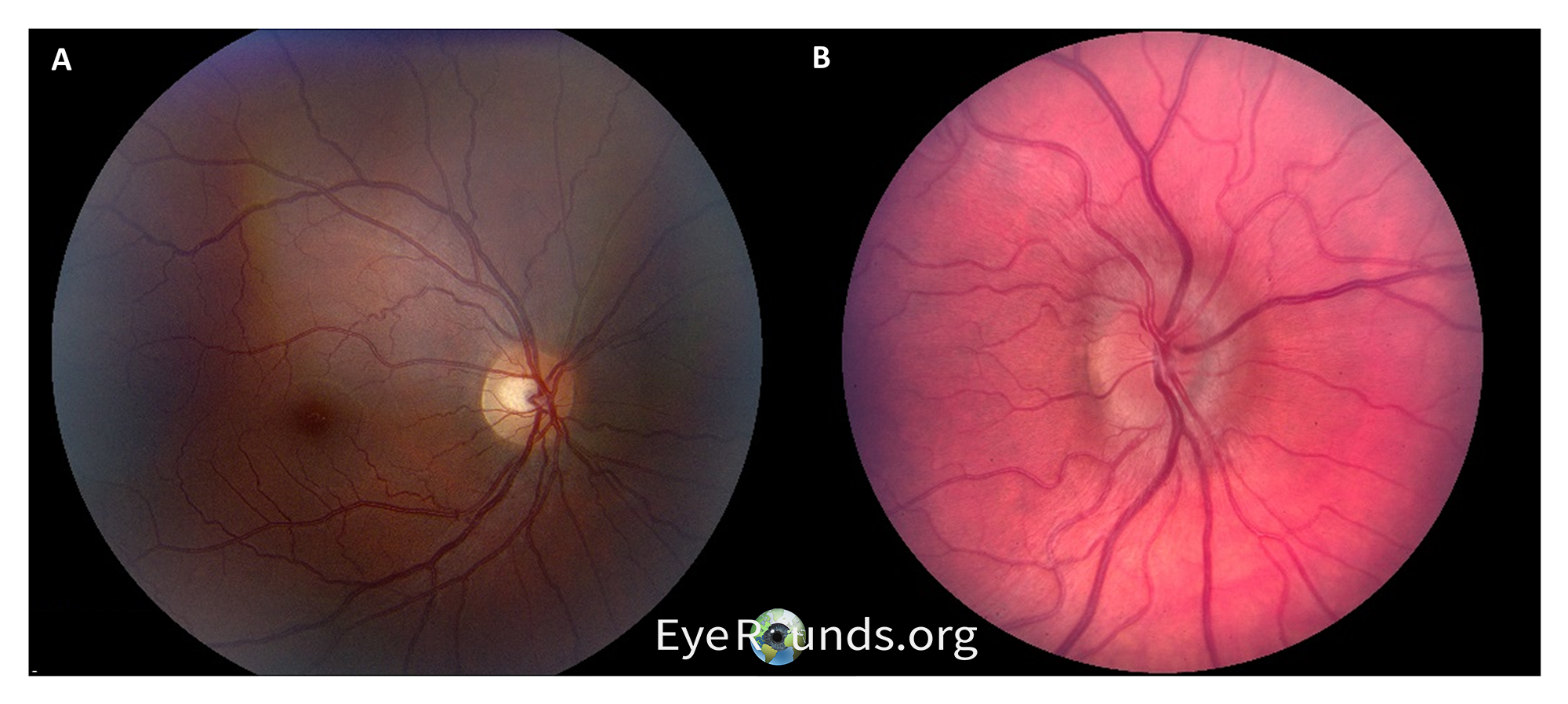
Papilledema is optic disc edema due to raised intracranial pressure and is ubiquitous in IIH, and an MRI with contrast is an important next step to evaluate for masses or other entities that could cause increased pressures. In cases of IIH, MRI should reveal normal brain parenchyma and ventricles. While multiple findings on MRI can occur in IIH, the most specific is posterior globe flattening (Sensitivity 43.5%, Specificity 100%). (7) With no secondary causes of increased intracranial pressure identified on imaging, the next step is a lumbar puncture (LP). It is important to note that an LP should be performed after imaging when findings are suggestive of increased intracranial pressures (such as papilledema) to avoid brain herniation resulting from a sudden decrease in pressures. Transtentorial brain herniation can occur when there is a mass lesion in one of the major brain compartments (e.g., the two hemispheric supratentorial compartments or the infratentorial compartment) and CSF pressure is lowered from below. The difference in compartmental pressures secondary to LP can therefore increase the risk for herniation. Opening pressures less than 200 mm H2O are considered normal and effectively exclude IIH while pressures greater than 250 mm H2O are considered elevated and provide further evidence (pressures of 200-250 are considered suspicious for ICP). Additionally, CSF studies should demonstrate no evidence of other abnormalities. Patients will occasionally report improvement in headache after an LP due to the removal of CSF and a subsequent decrease in intracranial pressures.
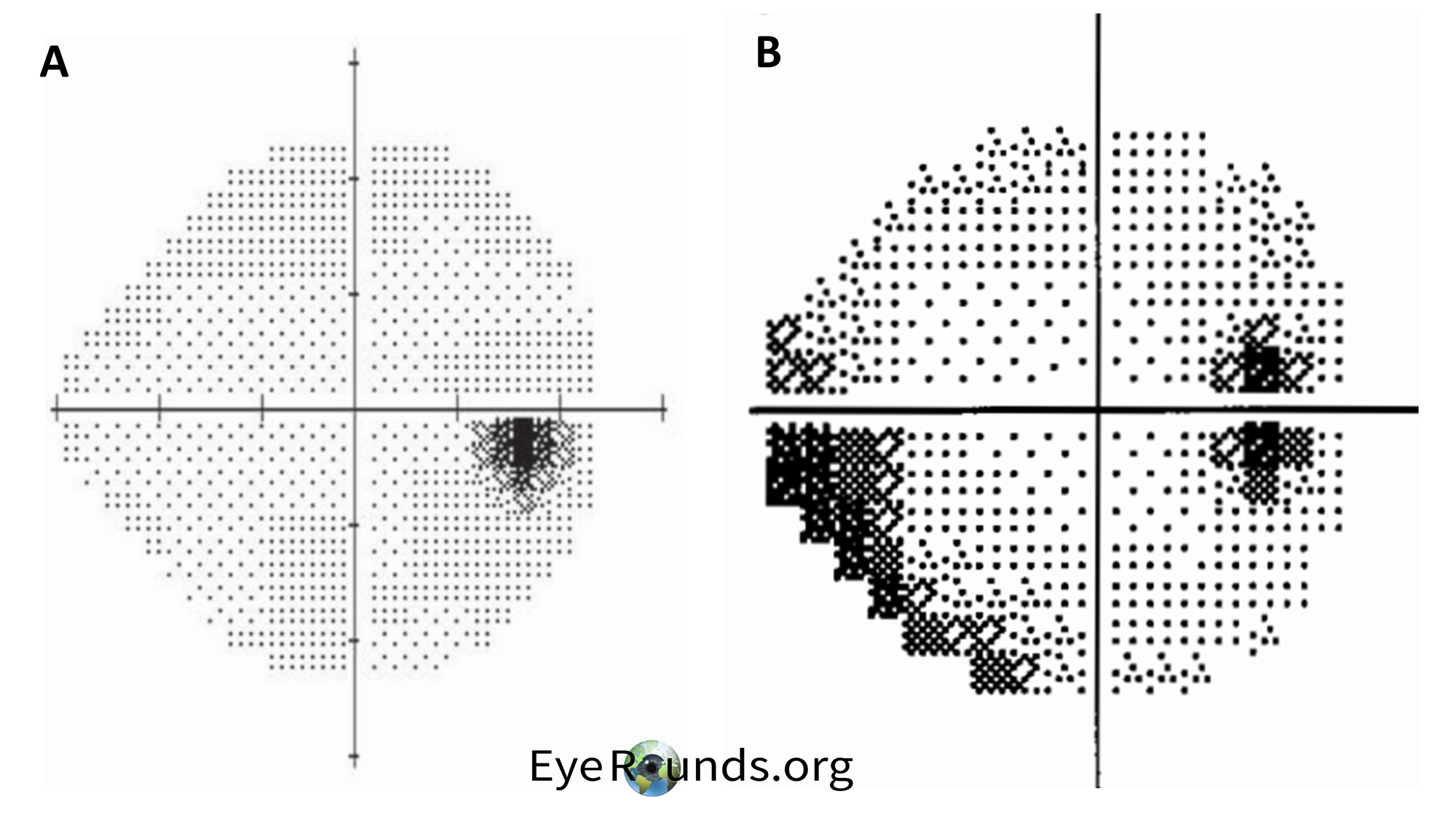
Visual field (VF) testing is also important as visual field loss is the primary morbidity associated with the disease. The characteristic finding in patients with IIH is an enlargement of the blind spot along with partial arcuate defects. (8) An example of these findings is shown below in Figure 4. For a refresher on visual field testing and interpretation, we recommend Visual Field Testing: From One Medical Student to Another (9). In brief, grayscale printouts are used to represent the sensitivity of the eye. Darker areas in a visual field result indicate worse vision in that region as illustrated in the inferior temporal section in Figure 4B (total deviation probability plots give a better indication of the extent of the visual loss). The optic disk is an important landmark in visual field results and is observed between 10° and 20° in the temporal visual field. Since the optic disk has no photoreceptors, it will appear dark on VF results.
Elevated intracranial pressures can lead to vision loss due to axoplasmic flow stasis at the optic disc. Prolonged and severely elevated intracranial pressures can compromise the intraneuronal blood supply and microcirculation within the nerve, along with the axonal transport of proteins resulting in nerve swelling (papilledema). Early on these changes are generally reversible; however, chronically increased pressures can lead to permanent visual field loss.
The treatment of IIH is largely based on the results of the IIHTT, the only successful major clinical trial for the treatment of IIH to date. Specifically, this study investigated the effect of acetazolamide compared to placebo in the treatment of IIH in patients concurrently receiving a low sodium weight-reduction diet. (10) Inclusion criteria for patients in the study were: age 18-60 with a diagnosis of IIH by modified Dandy criteria, reproducible visual loss with average perimetric mean deviation -2 dB to -7 dB in the eye with the greatest loss along with bilateral papilledema. (10) The results from this randomized, masked, placebo-controlled trial spanning more than 2 years showed that acetazolamide with a low sodium diet significantly improved perimetric mean deviation, papilledema grade, weight loss, and vision-related quality of life when compared to placebo in patients concurrently on a low sodium weight-reduction diet. (10)
Following the results of the IIHTT, first-line treatment of IIH includes weight loss—including bariatric surgery if indicated—and acetazolamide, a carbonic anhydrase inhibitor in the maximally tolerated dosage of up to 4 grams per day. Recall that carbonic anhydrase is an important enzyme in the production of CSF and its inhibition has been linked to decreased CSF production. Common side effects of acetazolamide include paresthesia and dysgeusia (especially a metallic taste with carbonated drinks). Topiramate, while not studied as rigorously as acetazolamide, is sometimes used if patients have contraindications to or unmanageable side effects from acetazolamide. Furosemide may be added to treatment regimens if initial treatment failure occurs. The treatment is similar for pregnant patients and acetazolamide can safely be started after 20 weeks gestation. Increased severity of papilledema (grades III-V compared to grades I and II), being male, and > 30 transient visual obscurations per month are all associated with a significantly increased risk of treatment failure, with male sex being the greatest risk factor. (11) Additionally, deterioration of the visual field is also significantly associated with weight gain in the year prior to diagnosis. (12) If a patient fails medical treatment and continues to have progressive visual loss, surgery with either optic nerve sheath fenestration or CSF shunting can be performed.
Vision loss typically worsens in the early stages of IIH before treatment initiation. However, treatment can help to stabilize decreases in vision, and in many cases partial or complete recovery of vision is possible. Long-term follow-up studies have shown that treatment leads to visual field improvement in most patients (60%) and bilateral blindness is a less common complication (10%). (12) IIH is a chronic condition that requires long-term monitoring and follow-up with weight surveillance, VF testing, and comprehensive ophthalmic exams.
Idiopathic intracranial hypertension is a chronic, progressive disease primarily affecting females between the ages of 20-50 years old who are overweight or obese, and its prevalence has been increasing due to the ongoing obesity epidemic. The most common symptoms of IIH include headache, transient visual obscurations, back pain, and pulsatile tinnitus. A comprehensive patient history and exam are important to rule out known causes of increased intracranial pressures including venous sinus thrombosis, vitamin A toxicity (including derivatives such as all-trans retinoic acid), tetracyclines, and excessive growth hormone. Important signs include papilledema, lumbar puncture opening pressures > 250 mm H2O, posterior globe flattening on MRI, and enlargement of the optic disk with inferior arcuate defects on perimetry. The major morbidity associated with IIH is visual loss. First-line treatment includes weight loss and acetazolamide to help reverse or stabilize visual field damage. Different interventions can be considered if the patients with severe, vision threatening papilledema or in patients that are refractory to more conservative therapies. Long-term follow-up and monitoring are required for all patients with IIH.
Both EK techniques require the use of a gas bubble into the anterior chamber to help position the graft against host tissue. EK, particularly DMEK, has a faster rate of vision recovery, better quality of vision, and a lower rejection rate than DSAEK. For this reason, DMEK is the preferred technique for corneal transplantation in the absence of complex anatomy (e.g., prior glaucoma surgery or prior retinal surgery).[6] DMEK surgical video available at EyeRounds.org (https://eyerounds.org/atlas-video/DMEK.htm). While visual outcomes post-transplant are typically quite good, there is always a risk of graft detachment, failure, or rejection, even years down the road. Some patients may require multiple transplants in their lifetime, and most will require prophylactic corticosteroid drops (i.e. prednisolone acetate 1%) for the rest of their life to prevent rejection.[7] Discussion on the identification and management of graft rejection and failure is beyond the scope of this article.
Heinzman Z, Wall MD. Idiopathic Intracranial Hypertension (Pseudotumor cerebri): From One Medical Student to Another. EyeRounds.org. May 3, 2024; Available from https://EyeRounds.org/tutorials/IIH-med-student/index.htm