


Ocular Vascular Clinic
Department of Ophthalmology & Visual Sciences
University of Iowa Carver College of Medicine
Iowa City, Iowa
Note: This is the second in a pair of articles about AION.
Part one is an introduction suitable for patients and others unfamiliar with AION.
Ischemic optic neuropathy constitutes one of the major causes of blindness or seriously impaired vision among the middle-aged and elderly population, although no age is immune. Its pathogenesis, clinical features and management have been subjects of a good deal of controversy and confusion. I have conducted basic, experimental and clinical research on the blood supply of the optic nerve and on various aspects of ischemic optic neuropathy since 1955. Based on those studies and other published reports, I have discussed the entire subject of ischemic optic neuropathy at length in a recent review article published in the journal Progress in Retina and Eye Research [74]. The following is an abbreviated version of that. For detailed information and bibliography, please consult the original paper. There is also a forthcoming book on AION by Sohan Singh Hayreh, it should be available in 2011.
Ischemic optic neuropathy is due to acute ischemia of the optic nerve.
Based on the pattern of blood supply of the optic nerve, it can be divided into two distinct regions:
Therefore, ischemic optic neuropathy is of two distinct types [17, 36, 74]:
Anterior ischemic optic neuropathy (AION): This is due to acute ischemia of the optic nerve head. Etiologically and pathogenetically, AION is of two types:
Posterior ischemic optic neurropathy (PION): [24, 60]: This is due to involvement of a part of the rest of the optic nerve. Etiologically and pathogenetically, PION is of 3 types:
NA-AION is the most common type of ischemic optic neuropathy, and has attracted the most controversy as to its pathogenesis and management.
This is discussed at length elsewhere [36, 74]. Following is a brief account:
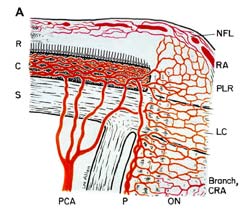
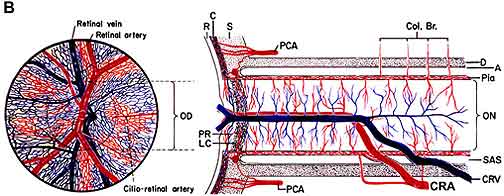
NA-AION is due to acute ischemia of the optic nerve head, whose main source of blood supply is from the posterior ciliary artery circulation (Fig. 1-A). Therefore, NA-AION represents an ischemic disorder of posterior ciliary artery circulation in the optic nerve head. Marked inter-individual variations in blood supply of the optic nerve head [25] and its blood flow [51] patterns profoundly influence the pathogenesis and clinical features of NA-AION.
Due to transient nonperfusion or hypoperfusion of the optic nerve head circulation: This is by far the commonest cause of NA-AION. There is almost a universally held belief among ophthalmologists and neurologists that NA-AION has a pathogenesis like that of a stroke which is a thromboembolic disorder; however, in the vast majority of NA-AION cases there is no evidence of that, as discussed at length elsewhere [74] - this is an extremely important fact to be borne in mind while managing NA-AION patients.
Naturally the question arises: what is the mechanism of transient nonperfusion or hypoperfusion of the optic nerve head circulation in NA-AION? It can be caused by a variety of factors. Available evidence indicates that in the vast majority of cases it is a transient fall of blood pressure, most commonly during sleep (nocturnal arterial hypotension - see below) or a nap during the day, or shock. A transient fall of perfusion pressure (perfusion pressure = mean blood pressure minus intraocular pressure) in the optic nerve head capillaries below the critical autoregulatory range (Fig. 2 below) in susceptible persons (see below), results in ischemia of the optic nerve head and development of NA-AION.
Due to embolic lesions of the arteries/arterioles feeding the optic nerve head: This is only an occasional cause of NA-AION. Compared to the hypotensive type of NA-AION, the extent of optic nerve head damage in this type is usually massive, severe, and permanent (similar to that in A-AION – see below), depending upon the size of the artery involved and the area of the nerve supplied by the occluded artery.

All the available evidence indicates that NA-AION is multifactorial in nature. The various risk factors fall into two main categories:
Predisposing risk factors make a person susceptible to develop NA-AION but do not necessarily produce NA-AION on their own. These may be systemic or local in the eye and/or optic nerve head.
The role of an absent or small cup in the pathogenesis of development of NA-AION: Since 1974, several studies have shown that in eyes with NA-AION there is a significantly higher prevalence of absent or small cup than in the general population [12, 72, 78]. This has resulted in a misconception in the ophthalmic community that a small or absent cup is actually the primary factor in the development of the disease; this has resulted in catchy terms like "disc at risk". The role of an absent or small cup in the pathogenesis of development of NA-AION is discussed in detail elsewhere [72, 78]. Briefly, it is evident that in the multifactorial scenario of pathogenesis of NA-AION, contrary to the prevalent impression, an absent or small cup is simply a secondary contributing factor, once the process of NA-AION has started, and not a primary.
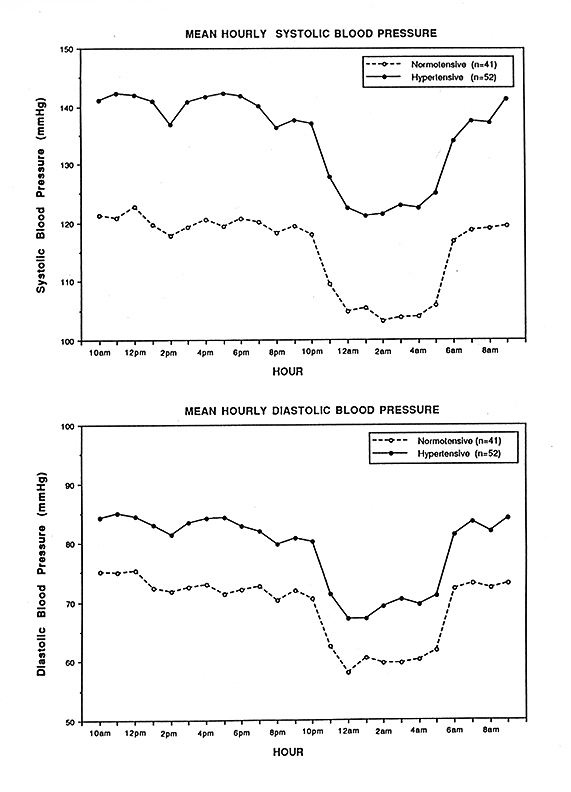
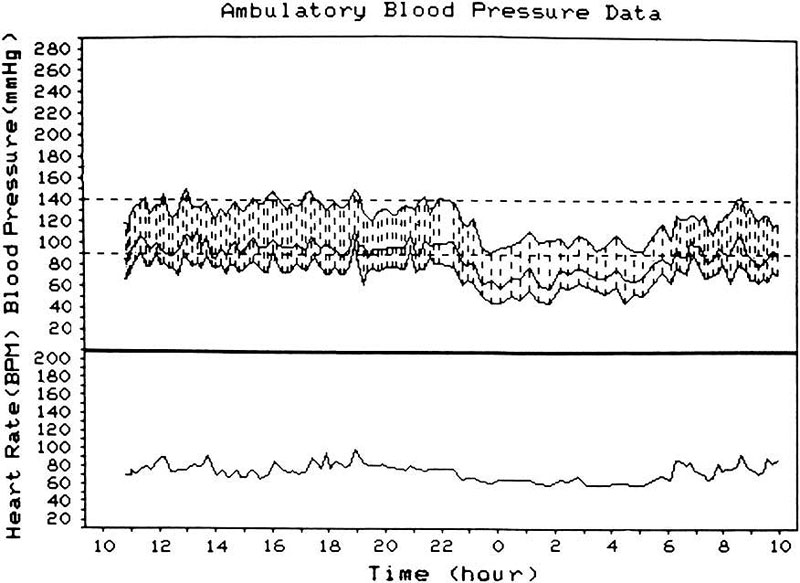
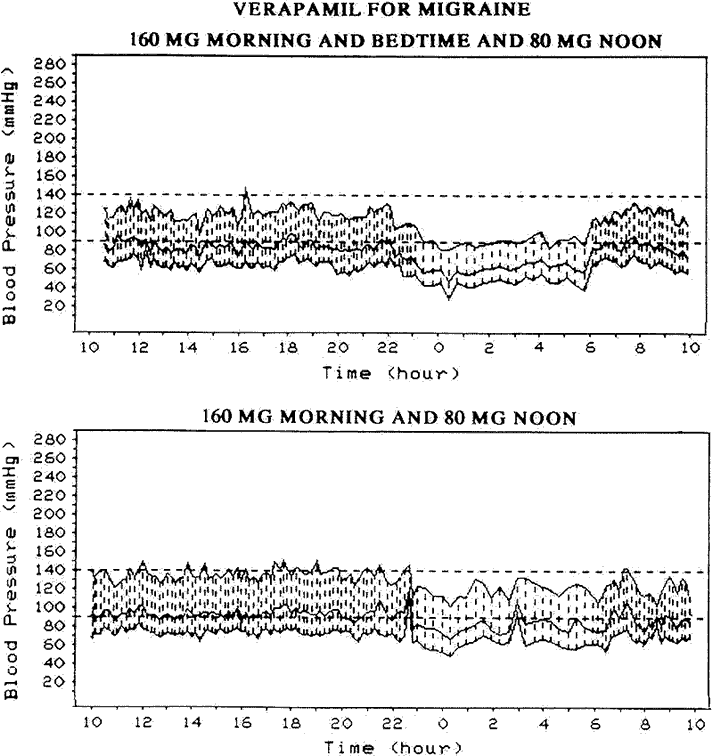
Precipitating risk factor(s): In a person with predisposing risk factor already present, these risk factors act as the final insult ("last straw"), resulting in ischemia of the optic nerve head and NA-AION. Nocturnal arterial hypotension is the most important factor in this category. This is because studies have shown that patients with NA-AION and often also those with A-AION typically complain of discovering visual loss on waking in the morning. In NA-AION, 73% gave a definite history of discovering the visual loss on waking up in the morning or from a nap, or first opportunity in the day to use vision critically [41]. The incidence may actually be much higher than 73% because among the remaining patients many were not certain when it had actually occurred. My 24-hour ambulatory blood pressure monitoring (Fig. 3 below) has shown development of marked nocturnal arterial hypotension in such patients. For example, the 24-hour ambulatory blood pressure monitoring pressure graph in figure 4 shows a steep drop in blood pressure on falling asleep at night and recovery to normal on waking in the morning. Studies have also shown that arterial hypertensives on oral hypotensive therapy have a significant association between progressive visual field deterioration in NA-AION and nocturnal hypotension [33, 46]. The fall of blood pressure during sleep is a physiological phenomenon, but it is influenced by many factors, including the various arterial hypotensive drugs taken for arterial hypertension or other cardiovascular disorders, particularly the number and amount of drugs taken and the time of day they are taken. When these drugs were taken at bedtime, they produced a far more marked degree of nocturnal hypotension than when taken in the morning, because they aggravate the naturally occurring fall of blood pressure during sleep (Fig. 5). There are, however, some patients who develop marked nocturnal hypotension even without any medication (presumably due to defective cardiovascular autoregulation), as can be seen in figure 4.
Conclusion: From this brief discussion, it becomes clear that development of NA-AION and the role of nocturnal hypotension in it, is highly complex. A whole host of systemic and local factors, acting in different combinations and to different extents may derange the optic nerve head circulation, with some making the optic nerve head susceptible to ischemia and others acting as the final insult. Nocturnal hypotension seems to be an important precipitating factor in the susceptible patient. It is the lack of in-depth understanding of the complex interrelationships that has resulted in controversy and confusion. The pathogenesis of NA-AION is complex but not, as often stated, unknown.
NA-AION is the most common type of ischemic optic neuropathy. It usually has classical symptoms and signs which make it easy to diagnose. The subject is discussed at length elsewhere [36, 74]. Following is a very brief account of the clinical features of NA-AION.
NA-AION is mostly a disease of the middle-aged and elderly, although no age is immune from it. In the vast majority, symptoms are typical. There is a sudden and painless deterioration of vision, usually discovered on waking in the morning [41]. When there is progressive visual loss, the patients again usually notice it on waking in the morning. NA-AION patients often complain of loss of vision towards the nose and less commonly altitudinal loss. Later on, photophobia is a common complaint, particularly in bilateral cases.
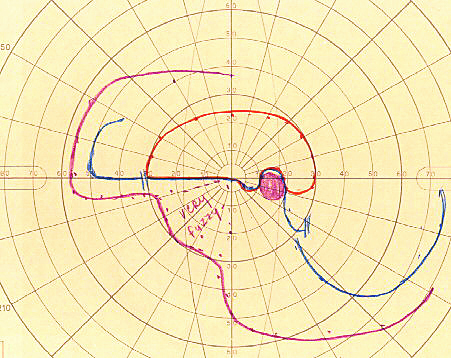
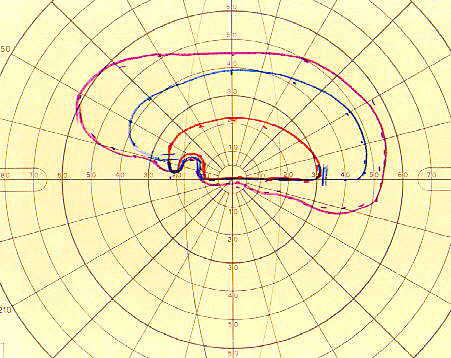
Poor visual acuity is common with NA-AION, however, initial visual acuity was 20/20 in 33%, better than 20/40 in 51% in a study of 500 consecutive NA-AION eyes [71, 73]. This shows that the presence of normal visual acuity does not rule out NA-AION - a common mistake. In contrast to that, visual field defects are a universal occurrence. Therefore, perimetry is the most important and essential visual function test to evaluate the visual loss. These eyes can present with a variety of optic nerve related visual field defects; however, a combination of a relative inferior altitudinal defect with absolute inferior nasal defect is the most common pattern in NA-AION (Fig. 6-A). This contradicts the commonly held belief that inferior altitudinal visual field defect (Fig. 6-B) is typical of NA-AION. My studies have shown that in NA-AION the visual field plotted with manual kinetic perimetry (using Goldmann perimeter) compared to that by automated perimetry provides far superior information about type of visual field defect and the peripheral field, and for evaluating visual functional disability [62]. This is because unfortunately, automated perimetry provides information on only up to about 24° - 30° in the periphery, whereas kinetic perimetry provides peripheral visual field information all the way to about 80° – 90° temporally, 70° inferiorly, 60° - 70° nasally and 50° - 60° superiorly.
Natural history of visual outcome in NA-AION: To evaluate whether a particular mode of treatment is beneficial or not, the first essential is to find out the natural history of a disease. It is not uncommon to find that natural history is credited as beneficial effect of a treatment. There are two prospective studies that have evaluated natural history of visual outcome in NA-AION [71, 79]; both arrived at the same conclusion. Both studies showed that in patients seen within 2 weeks of onset of visual loss and initial visual acuity of 20/70 or worse, there was spontaneous improvement of visual acuity in 41% - 43% and worsening in 15%-19% at 6 months. My study also evaluated visual fields with kinetic perimetry and that showed that 26% of those who were first seen <2 weeks of onset with moderate to severe visual field defect, showed improvement at 6 months [71]. Visual acuity and visual fields showed improvement or further deterioration mainly up to 6 months, with no significant change after that [71]. When NA-AION develops in the second eye, there is no correlation in the visual outcome in the two eyes.
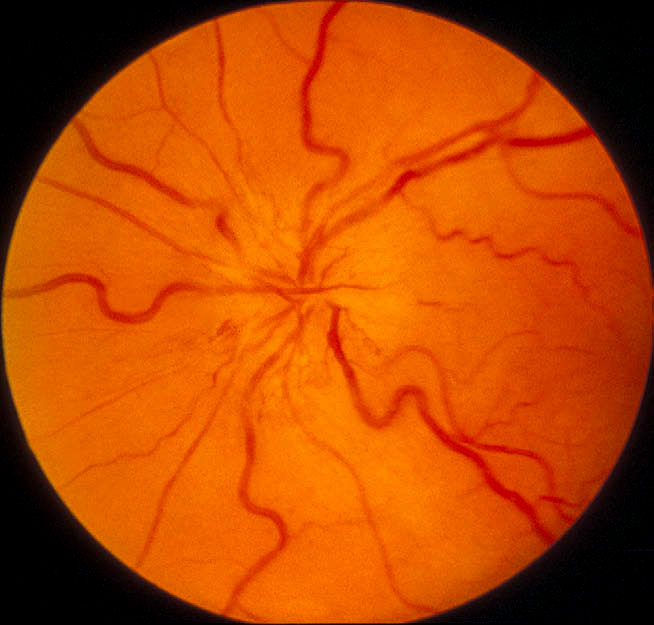
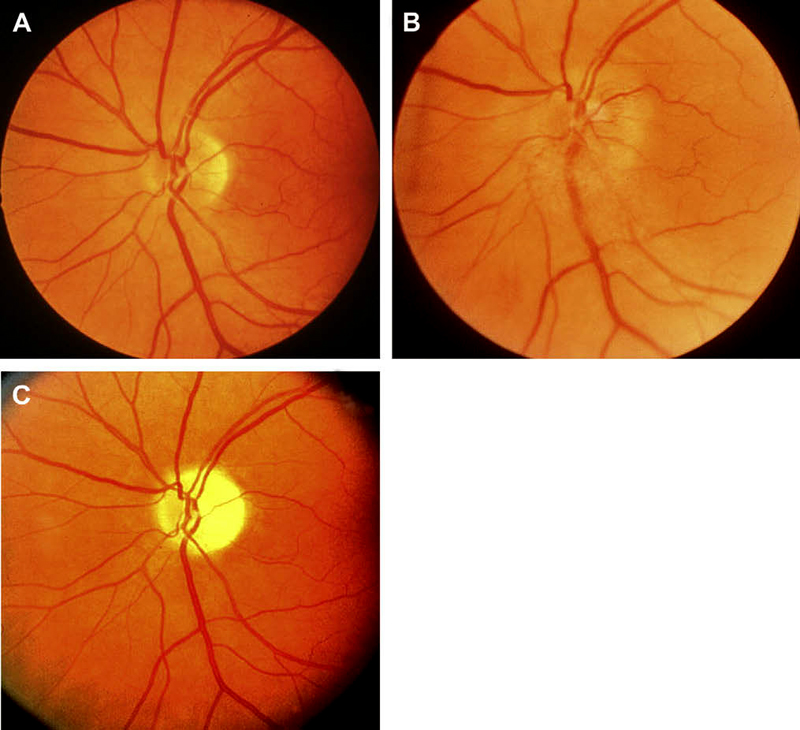
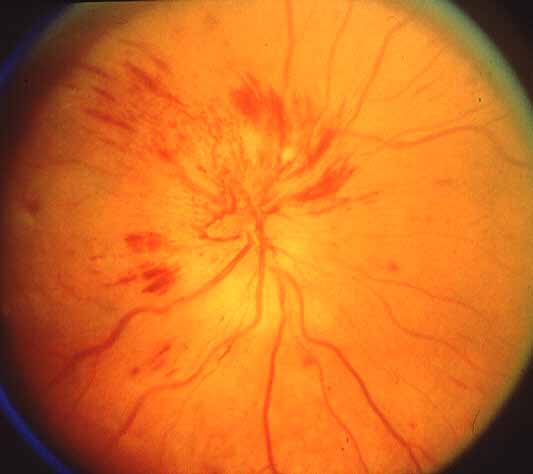
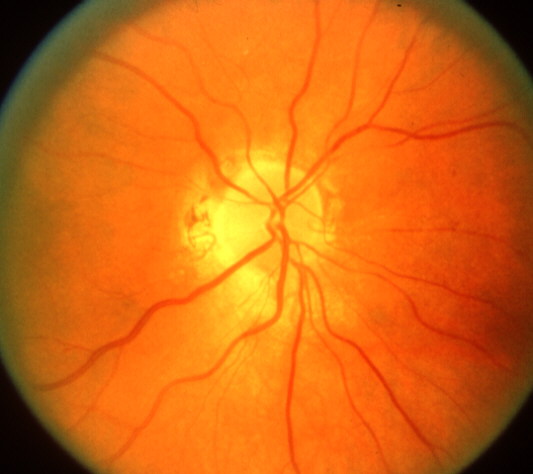
<Ophthalmic evaluation: At the onset of visual loss, there is always optic disc edema. There are several misconceptions about optic disc edema in NA-AION. The most common one is that in NA-AION the optic disc edema is always pale – that is not true at all initially, because the color of optic disc edema in NA-ION initially does not differ from optic disc edema due to other causes – in some cases there may even be hyperemia of the optic disc [66] (Figs. 7, 8, 9-B). A splinter hemorrhage at disc margin is common (Fig. 8). Optic disc edema starts to develop pallor about 2-3 weeks after the onset of NA-AION, and optic disc edema usually resolves spontaneously in about 2 months [66]. There is a characteristic evolutionary pattern of optic disc edema in NA-AION, as discussed elsewhere [66]. On resolution of optic disc edema, the distribution of optic disc pallor does not always correspond with the extent and location of visual and nerve fiber loss [66]. In occasional cases, where NA-AION is due to embolism, the optic disc edema usually has a chalky white appearance unlike in the classical NA-AION.
Ophthalmic evaluation: At the onset of visual loss, there is always optic disc edema. There are several misconceptions about optic disc edema in NA-AION. The most common one is that in NA-AION the optic disc edema is always pale – that is not true at all initially, because the color of optic disc edema in NA-ION initially does not differ from optic disc edema due to other causes – in some cases there may even be hyperemia of the optic disc [66] (Figs. 7, 8, 9-B). A splinter hemorrhage at disc margin is common (Fig. 8). Optic disc edema starts to develop pallor about 2-3 weeks after the onset of NA-AION, and optic disc edema usually resolves spontaneously in about 2 months [66]. There is a characteristic evolutionary pattern of optic disc edema in NA-AION, as discussed elsewhere [66]. On resolution of optic disc edema, the distribution of optic disc pallor does not always correspond with the extent and location of visual and nerve fiber loss [66]. In occasional cases, where NA-AION is due to embolism, the optic disc edema usually has a chalky white appearance unlike in the classical NA-AION.
In the fellow normal eye, optic disc usually shows either no cup or small cup (see "predisposing risk factors" above). This can be a helpful clue in the diagnosis of NA-AION in doubtful cases. If originally both eyes have a small disc cup, I have seen that in unilateral NAION, once the disc edema resolves, the cup in the involved eye may become slightly larger than the fellow eye because of loss of nerve fibers.
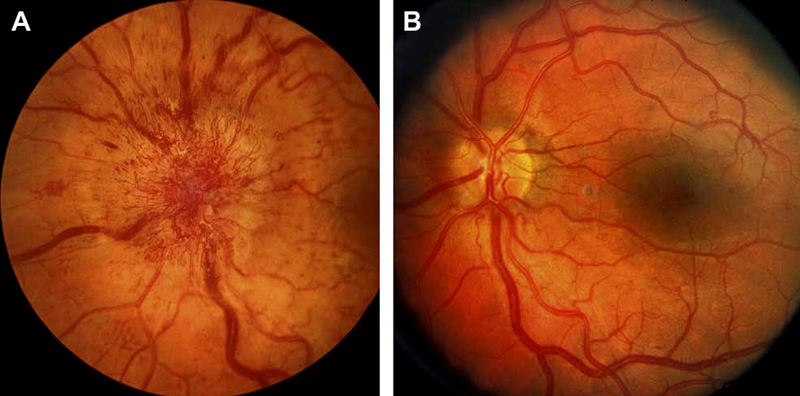
In diabetics, optic disc changes in NA-AION may have some characteristic diagnostic features. During the initial stages, the optic disc edema is usually (but not always) associated with characteristic prominent, dilated and frequently telangiectatic vessels over the disc, and much more numerous peripapillary retinal hemorrhages than in non-diabetics (Figs. 10-A, 11-A) [23, 70] These findings may easily be mistaken for proliferative diabetic retinopathy associated with optic disc neovascularization. When the optic disc edema resolves spontaneously, these prominent telangiectatic disc vessels and retinal hemorrhages also resolve spontaneously (Figs. 10-B, 11-B). The presence of these characteristic fundus changes in some diabetics with NA-AION has resulted in a good deal of controversy because it has been thought to be a separate clinical entity - described under different eponyms, the most common being "diabetic papillopathy", when in fact it is NA-AION [70].
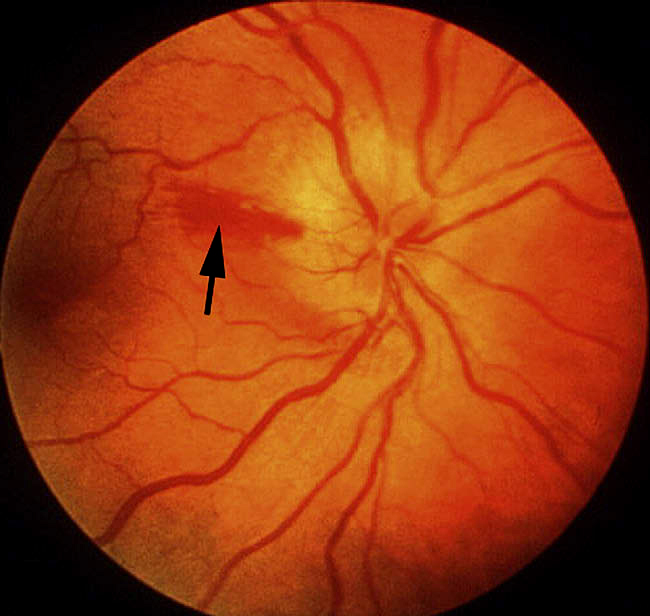
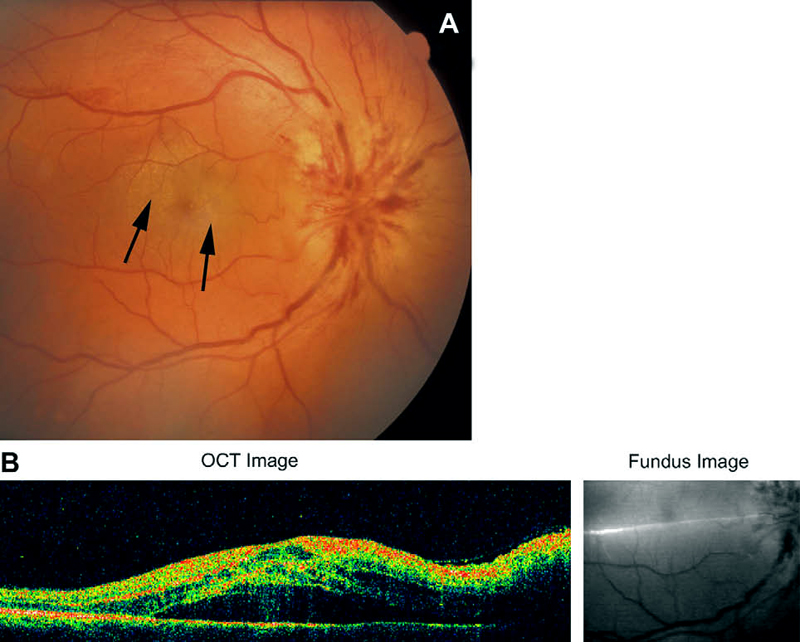
Other fundus changes: The presence of a few splinter hemorrhages on optic disc or immediate peripapillary region is common in association with the optic disc edema (Fig. 8); those resolve spontaneously with optic disc edema resolution. Diabetics tend to have more peripapillary retinal hemorrhages than non-diabetics [23, 70]. Occasionally, there may be mild serous retinal detachment between the optic disc and macula and that may even extend to macular region to produce macular edema (Fig. 12).
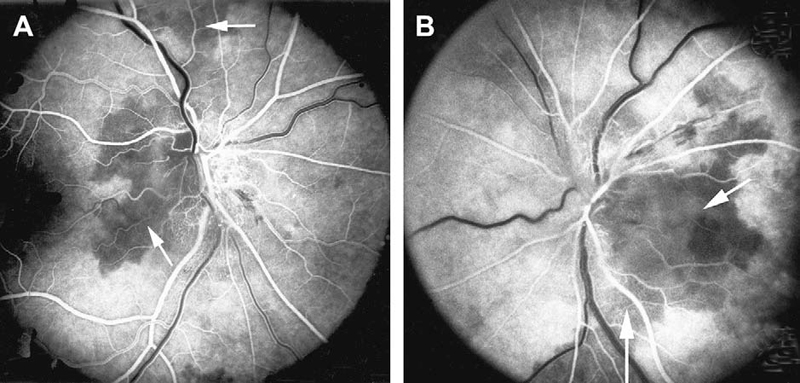
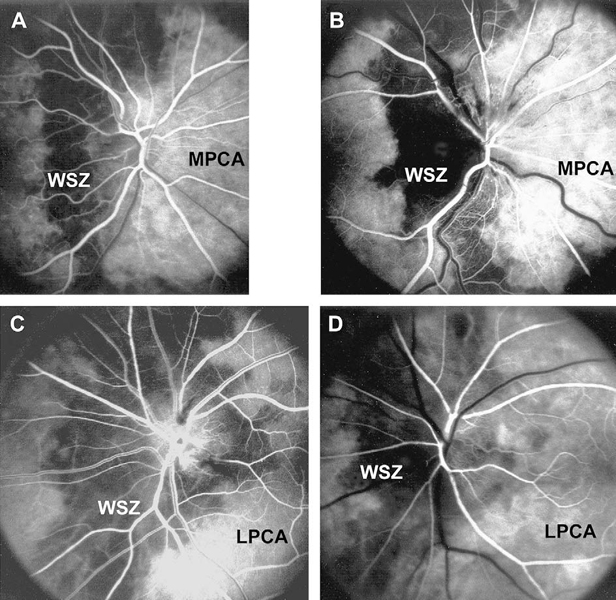
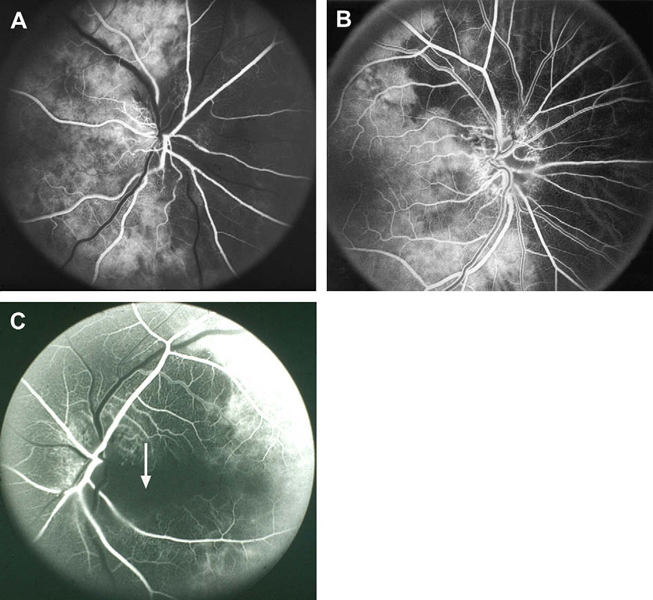
Fluorescein fundus angiographic findings: It is only when angiography is performed during the first few days after the onset of visual loss and during the very early arterial phase of dye filling in the fundus that demonstrates the tell-tale impaired circulation and its location in NA-AION. In my studies, there is almost invariably filling defect/delay in the prelaminar region and in the peripapillary choroid (Fig.13) and/or choroidal watershed zones (Figs. 14-A, B, D) at onset of NA-AION [25]. In the occasional case, where NA-AION is due to embolism into the posterior ciliary artery, the part of the choroid supplied by the occluded posterior ciliary artery or short posterior ciliary artery does not fill (Fig. 15-A). In view of that, fluorescein angiography provides very useful information when patients are seen early, particularly in differentiation of NA-AION and A-AION; therefore, it is essential to perform that in all early cases. Late optic disc staining is a non-specific finding of optic disc edema, and has no diagnostic importance for NA-AION.
Bilateral NA-AION: The cumulative probability of the fellow eye developing NA-AION has varied among different studies: 25% within 3 years [80], 17% in 5 years [81] and 15% over 5 years [4]; however, different criteria were used to determine the probability, which may explain the differences. According to one study [4], the risk is greater in men, particularly young diabetic men. The risk of the second eye getting involved by NA-AION is significantly greater in diabetics than in nondiabetics [70].
Recurrence of NA-AION in the same eye: In a study of 829 NA-AION eyes, the overall cumulative percentage of recurrence of NA-AION in the same eye was about 6% at two years [53]. The only significant association for recurrence of NA-AION was with nocturnal arterial hypotension. Thus, this study indicated that nocturnal diastolic arterial hypotension might be a risk factor for recurrence of NA-AION; however, since NA-AION is a multifactorial disease, other risk factors so far unknown may also play a role.
NA-AION and erectile dysfunction drugs: The subject is discussed at length elsewhere [61, 64]. Briefly, most patients who reported to have developed NA-AION following the use of these drugs are middle-aged or elderly men who generally already had various predisposing risk factors for NA-AION (see above). These drugs are mostly taken in the evening for sexual intercourse. They result in fall of blood pressure; when taken in the evening, as discussed above, there is high chance of them producing abnormal nocturnal arterial hypotension, which may be further aggravated if the person taking other arterial hypotensive drugs for arterial hypertension or other cardiovascular disorders. Like the vast majority of NA-AION patients, most of the patients reporting NA-AION following ingestion of these drugs discovered visual loss upon awakening in the morning. A critical review of all the reported cases shows a usually good temporal relationship between the ingestion of these drugs and onset of NA-AION. When all the above evidence is put together, it suggests that Viagra® (sildenafil) and other erectile dysfunction drugs can result in development of NA-AION in persons who already have predisposing risk factors.
Amiodarone and NA-AION: There is a universal belief that amiodarone causes optic neuropathy, called "amiodarone-induced optic neuropathy". However, various facts discussed elsewhere [63] show that this in fact is NA-AION. In the multifactorial scenario of NA-AION, it is the systemic cardiovascular risk factors rather than amiodarone that cause NA-AION.
Familial NA-AION: There are 5 reports in the literature representing 10 unrelated families in which more than one member developed NA-AION [67]. We have shown that this rare entity of familial NA-AION is clinically similar to the classical non-familial NA-AION, with the exception that familial NA-AION occurred in younger patients and had much higher involvement of both eyes than the classical NA-AION. The role of genetic factors in familial NA-AION is not known.
This has been a highly controversial subject. Over the years, a number of treatments have been advocated, including optic nerve sheath decompression, aspirin, systemic corticosteroids, and intravitreal triamcinolone and vascular endothelial growth factor inhibitory drugs. Optic nerve sheath decompression was found to be not only of no benefit but also a harmful procedure [79]. Following is a discussion of the currently advocated treatments.
A recent large, prospective study [73],reported its finding on the role of systemic corticosteroid therapy in NA-AION. In this "patient randomization" study, there were 696 eyes with NA-AION; of these cases, 51% voluntarily opted for systemic therapy while 49% opted for no treatment. There was no significant difference between the two groups in initial visual acuity and visual fields defects, and systemic diseases, except that patients who opted for treatment were slightly younger (59.2 vs. 62.0 years) and had a lower prevalence of arterial hypertension (34% vs. 43%). To determine if those factors influenced the visual outcome, they were accounted for in the statistical analysis by including them as covariates in the logistic regression model – they made no difference in visual outcome (age p=0.8; hypertension p=0.6).
Median follow-up was 3.8 years. At 6 months from onset of NA-AION, of the eyes with initial visual acuity 20/70 or worse and seen within 2 weeks of onset, there was visual acuity improvement in 70% (95% confidence interval (CI): 57.3%, 79.9%) in the treated group compared to 40.5% (95% CI: 29.2%, 52.9%) in the untreated group (odds ratio of improvement: 3.39; 95% CI:1.62, 7.11; p=0.001). Comparison of visual field defect at 6 months from onset of NA-AION, among those seen within 2 weeks of NA-AION onset with moderate to severe initial visual field defect, there was improvement in 40% (95% CI: 33.1%, 47.5%) of the treated group and 24.5% (95% CI: 17.7%, 32.9%) of the untreated group (odds ratio: 2.06, 95% CI: 1.24, 3.40; p=0.005). In both treated and untreated groups, the visual acuity and visual fields kept improving up to about 6 months from onset of NA-AION and very little thereafter.
Therefore, in NA-AION with no proven and effective treatment so far, this study suggested that treating these patients with systemic corticosteroids during the acute phase results in a significantly higher probability of improvement in visual acuity (p=0.001) and visual fields (p=0.005), compared to an untreated group. Both visual acuity and visual fields improved for up to 6 months after onset of NA-AION and no more after that.
Neuro-ophthalmologists and neurologists do not accept the findings of this study and are of opinion that corticosteroid therapy has no role in treatment of NA-AION. They have raised the following objections to this study:
Neuro-ophthalmologists and neurologists do not accept the findings of this study and are of opinion that corticosteroid therapy has no role in treatment of NA-AION. They have raised the following objections to this study:
The authors of this study have discussed all these objections at length in their paper. Following are the responses:
Naturally, the question arises, why did corticosteroid therapy help to improve the visual acuity and visual fields of NA-AION patients? This is discussed at length elsewhere[73].
Therefore, there are primary and secondary changes in the optic nerve head to produce optic disc edema in NA-AION - the primary change being ischemic axoplasmic flow stasis in the axons and the secondary vascular changes and fluid leakage.
Foulds [95] postulated that corticosteroid therapy in acute NA-AION reduces optic disc edema by reducing the capillary permeability. There is ample evidence that corticosteroids work in many non-inflammatory diseases. For example, a large number of studies have shown that corticosteroid therapy reduces macular edema due to various causes. It is due to reduction of capillary permeability and decrease of fluid leakage. As discussed above, fluorescein angiography shows leakage of fluorescein in the optic nerve head when the disc is edematous in NA-AION but not in normal or atrophic discs - a proof of increased capillary permeability in optic disc edema. A study investigated the effect of systemic corticosteroid therapy on optic disc edema in NA-AION, by comparing the rate of resolution of optic disc edema in the treated group (343 eyes) versus the untreated group (380 eyes) [66]. It showed that those treated with corticosteroid therapy within 2 weeks after onset of NA-AION had significantly (p=0.0006) faster optic disc edema resolution than the untreated cases. This indicates reduction in capillary leakage, similar to that seen in macular edema with corticosteroid therapy.
Thus, from the above discussion, the scenario that emerges to explain the beneficial effect of corticosteroid therapy on visual outcome in NA-AION seems to be as follows. The faster resolution of optic disc edema with corticosteroid therapy compared to the untreated patients [66] → progressive decrease of compression of the capillaries in the optic nerve head → better blood flow in the capillaries → improved circulation in the optic nerve head → improved function of the surviving but not functioning hypoxic axons. There is a possibility that corticosteroids may have beneficial effects from some other unknown mechanisms; one of those mentioned has been inhibition of damage by free radicals.
A well-known neuro-ophthalmologist, while commenting on the role of corticosteroid therapy in NA-AION, stated: "Oral steroids in the setting of acute cerebral stroke are contraindicated". This statement is based on a serious misconception about pathogenesis of NA-AION.[74] Cerebral stroke is a thromboembolic disorder, involving a large mass of tissue in the cerebrum. In contrast to that, NA-AION is a hypotensive disorder, involving a minute amount of tissue in the optic nerve head. To equate the two conditions is a fundamental mistake and responsible for confusion and controversy on various aspects of NA-AION, including its management.
The reason was discussed in the original paper.[73] In early 1970s, I planned a large, multicenter randomized clinical trial to investigate systematically in a large cohort of NA-AION patients whether systemic corticosteroids improved visual outcome. Unfortunately, that clinical trial was not funded by the NIH, because of a firm belief (based on no scientifically valid data) among neuro-ophthalmologists that corticosteroid therapy has no role in NA-AION. Since no alternative treatment existed, I felt that it was crucial to find out whether corticosteroid therapy was actually beneficial, ineffective or conceivably harmful in NA-AION. Lacking funding, I decided on a "patient choice" study instead of the "conventional randomized study" – the next best choice. Every NA-AION patient seen in my clinic was given a free and informed "patient choice". The decision was left entirely up to the patient, to opt for corticosteroid therapy or no treatment, in consultation with their physicians or other sources. We had no in-put at all into their choice. I specifically told all patients that I really did not know whether the treatment was beneficial, ineffective or even harmful. I collected the data for 28 years, completely masked about visual outcomes and numbers of patients in each group. The study finally included 613 consecutive NAION patients (696 eyes) seen in my clinic.[73]
What are the critical criteria for "conventional randomization"? It is to have comparable treated and untreated groups at baseline in demographic and clinical characteristics. In this study,[73] there was no significant difference between the treated and untreated groups in the baseline demographic and clinical characteristics, as is evident from the following:
Thus, this "patient choice" study fulfilled the most crucial criteria of any clinical trial, i.e. the treated and untreated groups were comparable in demographic and clinical characteristics. Therefore, the results of this study must be valid.
I expected this criticism from the start of the study. Therefore, all possible steps were taken in collecting all the data in a masked fashion, and during data analysis. These steps are discussed at length in the paper.[73]
This discussion, answers all the objections raised by critics to this study. In fact, all this information is already provided in detail in the original paper.[73]
Treatment protocol in this systemic corticosteroid therapy study
All patients were given initially 80 mg Prednisone daily for 2 weeks, then tapered down to 70 mg for 5 days, 60 mg for 5 days, then cut down by 5 mg every 5 days, to nothing.
Who, when and how to treat NA-AION patients with corticosteroid therapy?
The sooner the treatment is started, the better are the chances of visual improvement. That may be because the shorter the duration of axonal ischemia, the fewer axons are likely to be damaged permanently.
Over a period of almost five decades having treated several thousand patients with corticosteroid therapy for a variety of conditions, including giant cell arteritis, scleritis, uveitis, orbital myositis, retinal vasculitis and other conditions, I have found that the most effective way to use corticosteroid therapy is to hit hard at the beginning and then taper down. The major flaw in the way corticosteroid therapy has been given for NA-AION in some studies is "too small a dose, for too short a period". This timidity has led to the prevailing misconception that corticosteroid therapy does not help NA-AION
This is discussed at length elsewhere [96]. There is a common belief that NA-AION and cerebral stroke are similar in nature pathogenetically and in management. Under this misconception, aspirin is routinely advocated in NA-AION. Cerebral stroke is a thromboembolic disorder, involving a large mass of tissue in the cerebrum. By sharp contrast, NA-AION is a hypotensive disorder, involving a minute amount of tissue in the ONH. Thus, there is a fundamental difference between the two [74].
Two large studies have shown that aspirin has no long-term benefit of reducing the risk of NA-AION.[81, 82]. The fellow eye in NAION: report from the Ischemic Optic Neuropathy Decompression Trial Follow-Up Study.[79] These findings are not surprising since NA-AION is not a thromboembolic disorder but a hypotensive disorder and aspirin has no effect on the blood pressure or nocturnal arterial hypotension
There have recently been two contradictory reports on this therapy, one based on 3 patients showing no benefit [1] and another one based on 4 eyes claiming beneficial effect [83]; however, the claims of the latter study are not justified [68].
It is impossible to judge the effectiveness of intravitreal modes of treatment in studies containing only one to 4 eyes when 41% - 43% of NA-AION eyes show spontaneous visual acuity improvement. Most importantly, intravitreal injection in NA-AION eyes can be harmful. Optic nerve head circulation depends upon the perfusion pressure (mean blood pressure minus intraocular pressure). Intravitreal injection increases the volume in the eyeball, thereby resulting in a transient rise of intraocular pressure. In addition, there are many reports showing a substantial rise in intraocular pressure a few days or weeks after intravitreal triamcinolone. In NA-AION; with already precarious optic nerve head circulation, even a small rise in intraocular pressure for any reason can further compromise the circulation and result in further visual loss. Oral corticosteroid therapy for NA-AION by contrast, did not have that effect on intraocular pressure during a short-term treatment [73]. Thus, one cannot equate oral and intravitreal corticosteroid therapy in NA-AION.
There is an anecdotal case report claiming reduction of optic disc edema and visual improvement after an intravitreal injection of bevacizumab (Avastin®) 3 weeks after the onset of NA-AION in one eye [84].
Like intravitreal triamcinolone, there are problems following intravitreal injection of anti-VEGF drugs. This is discussed at length elsewhere.[93] Briefly, following intravitreal injection of Avastin, a short term and a long term sustained rise of intraocular pressure has been documented. As discussed above, blood flow in the optic nerve head in NA-AION is already precariously poor. Any rise of IOP can be harmful in such circumstances. I recently reviewed a manuscript where the authors treated 3 eyes with anti-VEGF therapy within 2 days after the onset of NA-AION and found no improvement in visual acuity and visual fields. Moreover, there are 2 reports of development of NA-AION following intravitreal injection of Avastin in eyes with age-related macular degeneration, because of vascular problems in persons of that age group.[93]
Thus, we have no evidence so far that intravitreal triamcinolone or anti-VEGF therapies have any beneficial effect. In fact, judging from the precarious circulation in the optic nerve head in NA-AION, these therapies, because of their associated rise in intraocular pressure, can be harmful.
The usual advice given by ophthalmologist and neurologists to NA-AION patients is that nothing can be done. Having dealt with more than a thousand patients with NA-AION and having investigated various aspects of NA-AION over the years, I find that is an inadequate and incorrect response. Firstly, because, as discussed above, systemic corticosteroid therapy during the early, acute stage of the disease has shown to be beneficial in visual outcome in a significant number of patients [73]. Secondly, as discussed above, NA- AION is a multifactorial disease and many risk factors contribute to it. The correct strategy is to try to reduce as many risk factors (discussed above) as possible to reduce the risk of NA-AION in the second eye or any further episode in the same eye.
As discussed above, nocturnal arterial hypotension is a major risk factor in NA-AION patients who already have predisposing risk factors. Since the 1960s many highly potent drugs with arterial hypotensive effect have emerged to treat arterial hypertension, other cardiovascular diseases, benign prostatic hyperplasia and other diseases; those drugs are currently widely used. It may not be coincidental that the incidence of NA-AION has progressively increased since the 1960s, so that it has now become a common visually disabling disease. This strongly suggests that NA-AION may be emerging as an iatrogenic disease, stemming from the aggressive use of the very potent arterial hypotensive agents now available. In view of this, management of nocturnal arterial hypotension seems to be an important step both in the management of NA-AION and in the prevention of its development in the second eye. Therefore, I strongly recommend that when a patient is at risk of developing ocular and optic nerve head ischemic and vascular disorders, or has the following:
In 1981, I reported [22] that "symptomless optic disc edema precedes the visual loss and may be the earliest sign of AION (NA-AION)". More recently, based on a detailed study of symptomless optic disc edema, I described this as a distinct clinical entity under the name of "incipient nonarteritic anterior ischemic optic neuropathy"[64]. This clinical entity initially presents with asymptomatic optic disc edema and no visual loss attributable to NA-AION. Available evidence indicates that it represents the earliest, asymptomatic clinical stage in the evolution of the NA-AION disease process; therefore, it shares most clinical features with classical NA-AION except for the visual loss.
These have been described in a recent study [64]. At initial visit, there is optic disc edema without any visual loss attributable to NA-AION (Fig. 16-A). In that study, incipient NA-AION progressed to classical NA-AION (after a median time of 5.8 weeks) in 25%, and 20% developed classical NA-AION after resolution of a first episode of incipient NA-AION. Patients with incipient NA-AION had a greater prevalence of diabetes mellitus than classical NA-AION; therefore, this has often been misdiagnosed as "diabetic papillopathy" or "diabetic papillitis", which has created confusion and controversy. Similarly, incipient NA-AION progressing to classical NA-AION has also been misdiagnosed as "amiodarone-induced optic neuropathy" in patients who happen to be on amiodarone therapy for cardiovascular disorders [63].
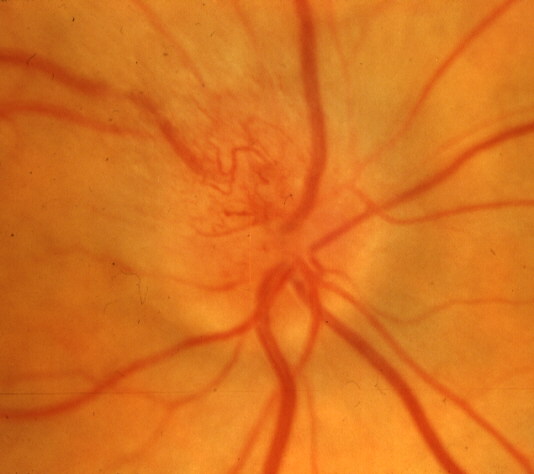
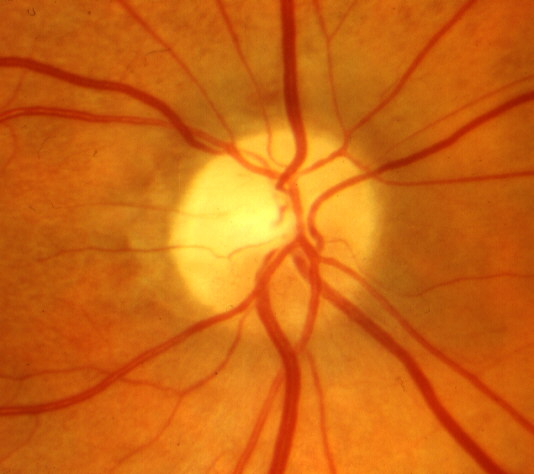
When a patient presents with asymptomatic optic disc edema, incipient NA-AION must be borne in mind as a strong possibility for those who have had classical NA-AION in the fellow eye, for diabetics of all ages, and for those with high risk factors for NA-AION. This can avoid unnecessary and expensive investigations.
To reduce the risk of progression of incipient to classical NA-AION, immediate steps should be taken to try to eliminate risk factors for development of NA-AION. These include nocturnal arterial hypotension, elevated intraocular pressure and evaluation for sleep apnea.
The subject of NA-AION is plagued with multiple misconceptions, resulting in controversy and confusion. Following are the major misconceptions.
More about fluorescein angiography
Blood tests and referrals to specialists in other areas: Blood tests which will be done immediately are the Erythrocyte Sedimentation Rate (ESR) and C-reactive protein (CRP). The results of these tests are available within a couple of hours. These two blood tests are extremely important to find out if a patient has giant cell arteritis. Both are usually abnormally high in giant cell arteritis.
Many other blood studies may be needed to find out if there is anything else wrong with the patient, and may help in finding the reason for the development of AION, such as diabetes or collagen vascular disease. Cholesterol and/or triglyceride levels will be checked because high levels lead to "hardening of the arteries." The results of these blood studies may indicate a need for follow-up by a local physician or referral to a specialist in hematology.
Blood pressure will be taken to determine if the patient has high blood pressure (hypertension). A cardiologist or cardiovascular specialist may be consulted if it seems that the cause of AION is a blood clot from somewhere else in the body, or that the heart, carotid arteries, or generalized "hardening of the arteries" may be contributing to the ischemia in the eye. Our recent studies have shown that abnormal fall of blood pressure during sleep is a serious risk factor for AION in the vast majority [12, 14, 15, 52, 60]. This can be tested by recording the blood pressure every 10 to 20 minutes over a 24 hour period, with an ambulatory blood pressure monitor
Temporal artery biopsy: When the patient's symptoms, the eye examination, elevated ESR and CRP, and fluorescein angiography make it seem likely that he/she has giant cell arteritis, a temporal artery biopsy will be done. The temporal artery lies just under the skin on the side of the forehead, or temple. The area is anesthetized and a small cut made in the skin to expose the artery. About an inch of the artery is removed for examination and the area is sutured with several stitches (which will be removed in about a week). This sample of the artery is examined under a microscope by a pathologist to determine whether there is inflammation of the artery. It may take several days before this result is known; however, treatment for giant cell arteritis may be started immediately if there is a strong suspicion of giant cell arteritis, even before the biopsy is done, because of the very high risk of blindness if adequate treatment is not given soon enough. Starting treatment before the biopsy is done does not interfere with the results.
Recent books by Dr. Sohan Singh Hayreh
Hayreh SS (2011). Ischemic Optic Neuropathies: Springer. 456 pages. ISBN: 978-3642118494
Hayreh SS (2015). Ocular Vascular Occlusive Disorders: Springer. 851 pages. ISBN: 978-3319127804