
Professor Emeritus of Ophthalmology
Ocular Vascular Clinic
Department of Ophthalmology and Visual Sciences
The University of Iowa
Roy J. and Lucille A. Carver College of Medicine
Please Note
Central retinal vein occlusion (CRVO) is a common cause of marked or total loss of vision in the middle-aged and elderly population, but no age group is immune to it. Although the disease entity has been known since 1878 and a large volume of literature has been published on the subject, its management is still ill-understood and controversial.
Since 1963 I have done experimental and clinical research on CRVO, its various types, course of the disease and various management options available and their merits and demerits. The following is a brief summary of my experience based on these studies and my way of managing CRVO
My studies showed that CRVO is actually of two types, with very different prognoses and management.[1-3] The two types are:
Classification of CRVO into non-ischemic and ischemic CRVO is essential because non-ischemic CRVO is a comparatively benign disease, with permanent central scotoma as the major complication from cystoid macular edema (see below). This type of CRVO does not develop the most dreaded complication of ocular neovascularization. In contrast to that, ischemic CRVO is a seriously blinding disease, and anterior segment neovascularization leading to neovascular glaucoma is its major complication (see below).
In our series of 620 consecutive CRVO cases, we have found that 81% of the patients have the non-ischemic type, and only 19% are ischemic type[3]. That is the fortunate part of the condition: only one fifth of the patients suffer the serious type.
The first crucial step in the management of CRVO is to find out what type of CRVO an eye has because the prognosis, management and outcome of the two are totally different.
Based on my experience of about 700 cases of CRVO over the years, I have developed diagnostic parameters to differentiate the 2 types of CRVO[1,2]. A prospective study of these parameters and their usefulness to differentiate ischemic CRVO from non-ischemic CRVO during the early phases of the disease revealed the following[2].
These tests can be divided into functional tests and morphological tests. Function tests consist of visual acuity, peripheral visual fields, relative afferent pupillary defect, and electroretinography. Morphological tests are: ophthalmoscopy and fluorescein fundus angiography.
Visual Acuity: In our study we found that a visual acuity of better than 20/200 (6/60) was seen in 58% of the non-ischemic, as compared to only 1.7% in the ischemic type of CRVO. A visual acuity of better than 20/400 (6/120) was seen in 81% of the patients of the non-ischemic, as compared to about 7% of the ischemic type of CRVO only. A visual acuity of 20/400 or worse was seen in only 19% of the non-ischemic, whereas it was seen in 93% of the ischemic type of CRVO. Thus, our studies showed that if an eye with CRVO has 20/400 or worse vision, then there is about a 90% chance that that eye has ischemic CRVO.
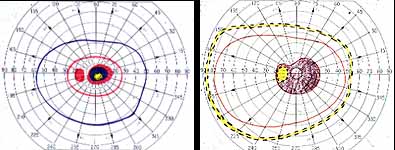
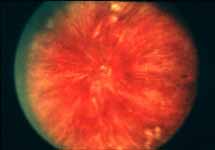
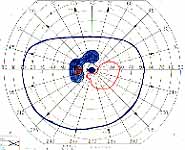
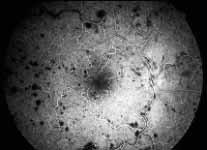
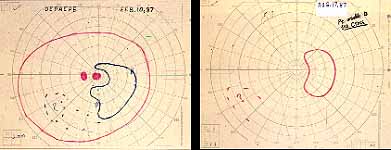
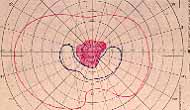
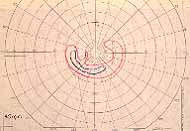
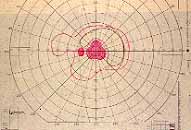
Peripheral Visual Fields: We find that peripheral visual fields, recorded with a Goldmann perimeter, give us very useful information in making this differentiation. Visual fields plotted with an automated perimeter do not help. We use, in the Goldmann perimeter, 3 isopters: I-2e, I-4e, and V-4e of the Goldmann perimeter. We find that in non-ischemic CRVO, the peripheral visual fields with V-4e and I-4e are perfectly normal, and they may even be normal with I-2e, or the I-2e is still seen in spite of the presence of peripheral visual field defects with this isopter. In ischemic CRVO, however, the eye may or may not see V-4e, or may or may not see I-4e, and usually does not see the I-2e target at all (Figure 1). Thus, in our study, in non-ischemic CRVO, all 3 targets were seen in 71% of the eyes, and I-4e and V-4e were seen in the remaining 29%, with no eye being unable to see any target or only V-4e. In contrast to that, in ischemic CRVO, only 8% of the eyes could see all the 3 targets, 63% saw I-4e and V-4e, and 18% only V-4e, and 10% could not see any target at all. Thus, if an eye can see I-2e or has normal visual fields with I-2e, that is definitely in favor of being a non-ischemic CRVO. If an eye can see only V-4e or no target at all, that eye definitely has ischemic CRVO.
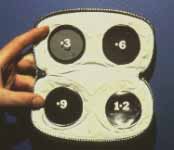
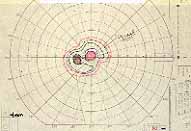
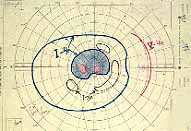
Relative Afferent Pupillary Defect (RAPD): The next test which helps us is the relative afferent pupillary defect. This is performed by a "swinging flashlight test". In a person with two normal eyes, if we shine the light on one eye, the pupil of that eye constricts immediately; then if we swing the light to the other eye, that pupil also constricts immediately. However, if one eye has retinal or optic nerve disorders, then if we first shine the light on the normal eye that pupil constricts, and then shine the light on the bad eye, instead of constricting the pupil dilates immediately in that eye; That is what is called a relative afferent pupillary defect. We can quantify the degree of relative afferent pupillary defect by using photographic neutral density filters (Figure 2). They come in 0.3, 0.6, 0.9 and 1.2 log units of transmission density, each 0.3 reducing the light by half. If we know that one eye has a relative afferent pupillary defect and the other eye is normal, then this filter is placed in front of the normal eye, not the bad eye. We start with 0.3 log unit filter in front of the normal eye. If on doing the test, the pupil in the bad eye still dilates, then we go to the next filter, 0.6 log unit. If it still dilates we go to the next filter, 0.9 log unit, and after that to the 1.2 log unit filter. (In fact, we can combine these filters to get higher log units.) So we keep doing that until the pupil in the bad eye starts to constrict instead of immediately dilating. That gives us the degree of the relative afferent pupillary defect.
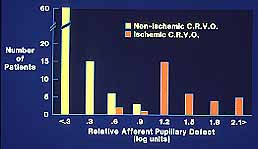
In our study we found that in non-ischemic CRVO, 97% of the eyes had a relative afferent pupillary defect of <0.6 log units. In contrast to that, in ischemic CRVO, 94% of the eyes had a relative afferent pupillary defect of >0.9 log units and 91% of the eyes >1.2 log units (Figure 3). Our studies also showed that all eyes with ocular neovascularization had relative afferent pupillary defects of >1.2 log units.
Electroretinography: We found that the electroretinography (ERG) parameter with the best sensitivity and specificity to differentiate ischemic from non-ischemic CRVO was amplitude of the b-wave (in both photopic and scotopic ERG). If the amplitude of b-wave is <60% of the normal, then there is an 80% chances that we are dealing with an ischemic CRVO. Or, if the amplitude of the b-wave is reduced by one or more standard deviation of the equipment, then we have a high chance of that being an ischemic CRVO. The advantage of ERG is that we can do this test even if the other eye is not perfectly normal, or in patients with only one eye. Relative afferent pupillary defect can only be tested when there is a perfectly normal second eye.
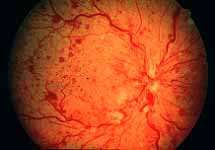
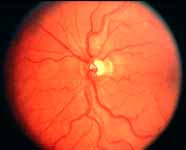
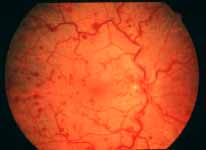
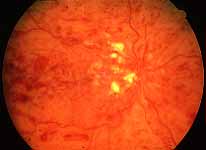
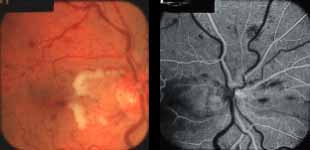
Ophthalmoscopy: Most ophthalmologists tend to use this as the main criterion to find out the type of CRVO. It is usually felt that extensive retinal hemorrhages and cotton-wool spots suggest an ischemic CRVO. However, our experience and our studies showed that these are very unreliable parameters. In fact our studies have shown that ophthalmoscopy is the least reliable and most misleading test for such a differentiation. For example, the eye in Figure 4 ophthalmoscopically looks like non-ischemic CRVO, but by all other parameters, it was an ischemic CRVO. Similarly, the fundus appearance of the eye in Figure 5a, with massive hemorrhages and few cotton-wool spots, is highly suggestive of ischemic CRVO; but the visual field plotting in Figure 5b shows that the eye can still see I-2e, and has normal peripheral visual fields with I-4e. As I mentioned earlier, if an eye can see I-2e there is a very high chance that that eye has non-ischemic CRVO. Moreover, this eye could see almost 20/40 - 20/50 which is another parameter telling us that this eye is a non-ischemic CRVO, in spite of extensive hemorrhages and some cotton-wool spots, which are considered signs of an ischemic CRVO. Two years later, the fundus and the angiographic appearances were perfectly normal in that eye - Figure 6a shows the fundus is perfectly normal, and Figure 6b shows fluorescein fundus angiogram with no capillary obliteration and perfectly normal retinal vascular beds; Thus, in fact this was a non-ischemic CRVO, which ophthalmoscopy would have made us believe was ischemic CRVO. Thus, our study has shown that the ophthalmoscopic appearances can be similar in ischemic and non-ischemic CRVO, during both early and late stages of the disease, and can mislead us in diagnosis.
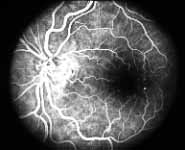
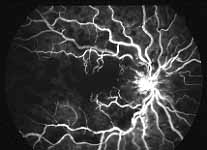
Fluorescein Fundus Angiography: In fluorescein fundus angiography, typically the retinal capillary non-perfusion or obliteration is considered the diagnostic criterion of ischemic CRVO. Figure 7a is a fundus photograph and Figure 7b an angiogram of an eye with non-ischemic CRVO, and typically we see that all the capillary bed fills very well, and there are a few scattered retinal hemorrhages and engorged retinal veins.
In contrast to that, in Figure 8 there is almost total non-perfusion of the retinal capillary bed, indicating that this is an ischemic CRVO. If such good-quality angiogram of non-ischemic and ischemic CRVO is available, then fluorescein fundus angiography is very helpful. Unfortunately, there are multiple limitations in the evaluation of retinal capillary non-perfusion by fluorescein fundus angiography in CRVO, especially in fresh cases. These limitations include:
Thus, our study showed that fluorescein fundus angiography provided information on retinal capillary non-perfusion in only 2/3 of the eyes during the acute phase because of various limitations listed above. Not only that, but also fluorescein fundus angiography provided misleading information in some. Therefore, in our study, overall, angiography performed much worse than any of the functional tests. No doubt, if we can get good-quality retinal capillary information from all over the retina, fluorescein angiography is the best test.
Fallacy of using "10 disc area of retinal capillary obliteration" as the criterion to define ischemic CRVO: Another very important misleading factor in the various studies in the literature differentiating ischemic from non-ischemic CRVO is the widely advocated use of a "10 disc area of retinal capillary obliteration" as the normal yardstick. Our studies have shown that this is usually an invalid criterion for diagnosis of ischemic CRVO by fluorescein angiography. A recent multicenter study4 on CRVO came to the same conclusion. The study results clearly showed that eyes with less than 30 disc diameters of retinal capillary nonperfusion and no other risk factor are at low risk for developing iris/angle NV, "whereas eyes with 75 disc diameters or more are at highest risk". Thus "10 disc area of retinal capillary obliteration" is a poor and unreliable parameter in differentiating ischemic from non-ischemic CRVO as well as predicting ocular NV; and its use in many studies has resulted in misleading information and confusion.
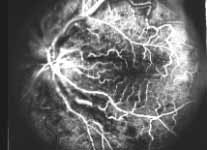
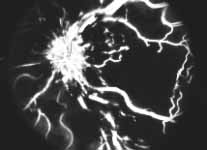
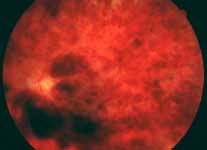
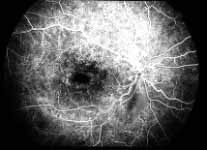
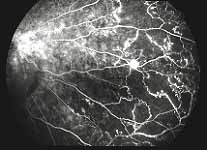
Table 1: The following table summarizes the sensitivities and specificity of the various functional tests.[2] |
|||
Functional tests |
Sensitivity |
Specificity |
|
Visual acuity: |
|||
< 6/120 |
91% |
88% |
|
Peripheral visual fields (Goldmann): |
|||
No I-2e |
97% |
73% |
|
Defective I-4e |
92% |
87% |
|
RAPD: |
|||
> 0.9 log units |
80% |
97% |
|
ERG: |
|||
b-wave amp.<60% |
80% |
80% |
|
Based on our study[2] (Table 1), for differentiation of ischemic from non-ischemic CRVO during the acute phase, we found the overall order of reliability of these tests as follows:
A study without a clear distinction between the ischemic and the non-ischemic CRVO has little clinical or scientific validity, because, as I mentioned earlier, non-ischemic CRVO is a comparatively benign condition and there is no risk of neovascularization in this type of CRVO. In contrast to that, ischemic CRVO is a severely blinding disease, with a high incidence of anterior segment neovascularization, particularly, neovascular glaucoma.
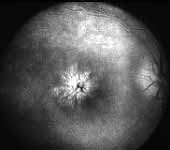
NON-ISCHEMIC CRVO: Long-term blinding complications are rare in this type. The major complication is chronic macular edema (Figure 13), leading on to cystoid macular degeneration, and permanent central scotoma; however, the peripheral visual field always remains normal. In about 12% of the eyes, within 18 months of onset non-ischemic CRVO may change to ischemic CRVO - more often in older than in younger persons[3]. If an eye with non-ischemic CRVO has a cilioretinal artery, then that eye develops cilioretinal artery occlusion (Figure 14) and an associated visual field defect and may produce sectoral optic atrophy.
ISCHEMIC CRVO: There are many serious complications in this type. The most important complication is the development of ocular neovascularization, with neovascular glaucoma as the most dreaded complication. It is seen only in ischemic CRVO, and the overall incidence in our series was about 45% among the ischemic CRVO eyes. Other complications of ischemic CRVO are vitreous hemorrhage, macular degeneration, optic atrophy, retinitis proliferans, phthisis bulbi or loss of eye.
Ocular neovascularization[5]: As I mentioned earlier, 81% of the patients with CRVO have the non-ischemic type, and only 19% have the ischemic type, and ocular neovascularization attributable to CRVO does not occur in non-ischemic CRVO; There are, however two exceptions to this: i.e. eyes with associated (a) ocular ischemia and (b) background diabetic retinopathy, because some of these eyes, in spite of having non-ischemic CRVO, may develop ocular neovascularization. In ischemic CRVO the neovascularization is mainly of the anterior segment. Figure 15 is a graphic representation of cumulative chances of developing various types of neovascularization (NV) in ischemic CRVO.
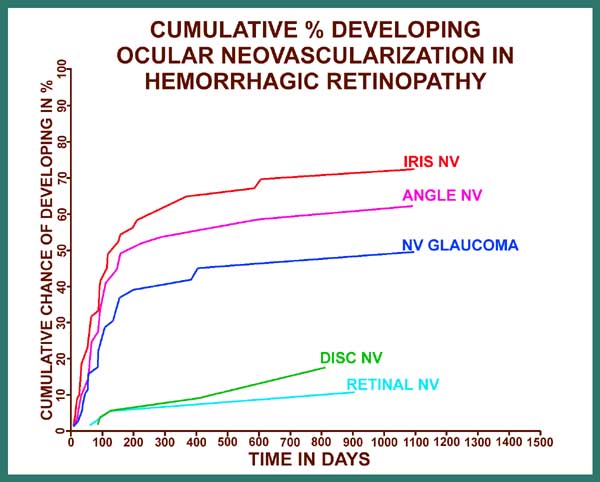
On the X axis for figure 15 are the days from the onset of ischemic CRVO, and on the Y axis is the cumulative chance or probability of developing neovascularization. The various graphs are, from above down, for iris neovascularization, angle neovascularization, neovascular glaucoma, disc neovascularization and retinal neovascularization. These graphs provide very important information on a number of issues, including the following:
In order to place ocular neovascularization in overall CRVO in true perspective, it is important to point out two important facts: (i) Ischemic CRVO constitutes only one fifth of all CRVO cases. (ii) neovascular glaucoma, the most dreaded complication of CRVO, is seen at the maximum in about 45% of ischemic CRVO cases only. This would indicate that the overall incidence of neovascular glaucoma in the entire CRVO group is only 8 - 10% at the maximum - a highly important fact in any management considerations for CRVO.
Vitreous hemorrhage: In CRVO this may be either secondary to retinal/optic disc neovascularization or due to rupture of the blood through the internal limiting membrane, particularly in eyes with many sub-internal limiting membrane hemorrhages. In my experience, the latter source of vitreous hemorrhage is much more common than the former, and can occur in non-ischemic as well as ischemic types of CRVO, usually during the early stages of the disease. Vitreous hemorrhage from retinal/disc neovascularization usually occurs during the late stages of the disease. Sometimes the vitreous hemorrhage can occur from intraretinal microvascular abnormalities secondary to CRVO or development of posterior vitreous detachment. Therefore, it is important to be aware of the fact that the presence of vitreous hemorrhages in CRVO does not always mean the presence of retinal/disc neovascularization.
For proper management of a disease, understanding the natural history of the disease is absolutely essential, so that the natural history may not be interpreted as a beneficial effect of the treatment being advocated. I have discussed elsewhere the following aspects of natural history of CRVO.[6]
| Table 2: Final visual acuity after resolution of retinopathy in my natural history study[10] | |
Visual acuity |
Non-ischemic CRVO |
20/15 to 20/40 |
65% |
20/50 to 20/80 |
9% |
20/100 to 20/200 |
11% |
20/400 |
8% |
CF or worse* |
7% |
Total eyes |
144 |
* = Main cause of poor vision other than retinopathy, e.g., cataract, macular degeneration, glaucoma, etc. |
|
CF = Counting fingers |
|
Management of CRVO still remains uncertain and highly controversial. Over the years many treatments have been advocated enthusiastically and success claimed but none has stood the test of time. These include anticoagulants, hemodilution, corticosteroids, acetazolamide, fibrinolytic agents, low molecular weight dextran infusion, carbon dioxide inhalation, vasodilators, hyperbaric oxygen, ocular hypotensive therapy, surgical decompression of CRVO, laser-induced chorioretinal anastomoses, photocoagulation, and a host of others.
The most important consideration when evaluating a therapy for any disease is to determine whether the therapy is based on incontrovertible scientific facts. Treatments without such a logical foundation prove not only useless but also sometimes harmful. Recently I have reviewed the various advocated modes of treatment in CRVO and their scientific validity [6] This review revealed that, unfortunately, most of the advocated treatments lack an incontrovertible scientific basis. Successes and beneficial effects claimed for many therapies in most cases simply represent the natural history of the disease, and that basic fact has been ignored in almost all the studies.
The fact that I would like to stress at the outset is that ischemic CRVO is a different, far more serious and potentially blinding disease than non-ischemic CRVO, and it requires wholly different management. Therefore, we must at first know which type of CRVO we are dealing with, and that makes differentiation of two types of CRVO the first essential step in the management of CRVO.
Following is a brief account of the major therapies advocated and tried from time to time. The main treatments can be divided into three categories: (A) medical, (B) surgical and (C) photocoagulation.
Complications: Various studies have reported a number of complications which can be divided into immediate and late complications.
To place the value of laser-induced chorioretinal venous anastomosis for treatment of nonischemic CRVO in true perspective, one has to consider the natural history of nonischemic CRVO (see "Natural history of CRVO" above) .
When evaluating the validity of claims of improved visual acuity in any study in the literature, one has to be aware of the possibility that apparent "improvement" in visual acuity can simply be the result of multiple artifacts in visual acuity testing (which is often done by technicians). In my experience of repeated testing of visual acuity, myself, in about three thousand patients with various circulatory disorders of the eye (e.g., anterior ischemic optic neuropathy, central and branch retinal artery occlusion and central and branch retinal vein occlusion, ocular ischemia) over time, I have found that the most important factor in apparent "improvement" of visual acuity may simply be a patient's improved skill in reading the visual acuity chart; he/she may have learned by experience to read the test chart better by looking around and fixating eccentrically. This applies particularly to an eye which has a visual field defect or a scotoma passing through or just involving the central fixation spot. (Eyes with nonischemic CRVO invariably have poor visual acuity due to central scotoma.) That is why reports of improved visual acuity without corresponding improvement of central visual field defects can be misleading.21 None of the studies reported concurrent improvement of visual acuity and visual field.
It is therefore evident from the available data that the visual outcome apparently achieved in eyes with nonischemic CRVO by laser-induced chorioretinal venous anastomosis seems no better, if not worse, than the natural history of the disease. On the top of that, the procedure is associated with a fairly high risk of serious vision threatening complications, not seen in non-ischemic CRVO
Conclusion: It is evident that the complications of laser-induced chorioretinal venous anastomosis for treatment of non-ischemic CRVO heavily outweigh any dubious benefits, and that this is not a safe and effective mode of treatment for a condition which has a fairly good outcome if simply left alone (see "Natural history of CRVO" above).
In the management of CRVO, panretinal photocoagulation has been widely advocated as the treatment of choice. The presumed role of panretinal photocoagulation in retinal diseases is to prevent development of neovascularization in eyes with retinal ischemia.
Non-ischemic CRVO: Since there is no retinal ischemia in this type of CRVO, and thereby no risk of developing neovascularization[5], there is no scientific basis at all for doing panretinal photocoagulation in non-ischemic CRVO. Moreover, panretinal photocoagulation is not a harmless procedure; it can produce loss of peripheral visual fields in CRVO (see below - Figures 16,17) and may cause defective vision in the dark. Some ophthalmologists advocate the use of macular grid photocoagulation for microcystic macular edema; however, a recent multicenter CRVO photocoagulation study revealed that it has no beneficial effects in these eyes and does not improve visual acuity[22]. In my experience, some patients may land up with much worse central scotoma from such a treatment. Thus, photocoagulation has no role whatsoever in the management of non-ischemic CRVO.
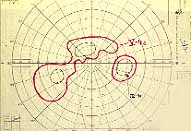
Ischemic CRVO: It has been almost universally accepted that prophylactic panretinal photocoagulation is the treatment of choice to prevent neovascular glaucoma or treat neovascular glaucoma itself. I have found little definite scientific proof in support of this assumption[23]. Therefore, we investigated this in a 10-year prospective study, to find out its effect on ocular neovascularization, particularly neovascular glaucoma associated with ischemic CRVO. The results, which were very surprising, were published in 1990[23]. In summary, our study showed that, on comparing the lasered eyes versus the non-lasered eyes, there was no statistically significant difference between the two groups in the incidence of development of: (a) angle neovascularization, (b) neovascular glaucoma, (c) retinal and/or optic disc neovascularization, and (d) vitreous hemorrhage, and (e) in visual acuity. Our study did show a statistically significant (p=0.04) difference in the incidence of iris neovascularization between the two groups, with iris neovascularization less prevalent in the lasered group than in the non-lasered group, but only when the laser was performed within 90 days after the onset of CRVO; however, iris neovascularization per se produces no harmful effect in the eye and as such is of little importance. The most important result of this study was the markedly deleterious effect of panretinal photocoagulation on the peripheral visual fields, because the lasered group suffered a significantly (p<0.03) greater loss than the non-lasered group (Figures 16, 17). Thus, contrary to the prevalent impression, our study showed that panretinal photocoagulation has no statistically significant beneficial effect in reducing neovascular glaucoma. On the other hand, it markedly damages the peripheral visual field, and may convert an eye with normal peripheral vision into almost a blind eye.
see also Figure 17. Visual Field Examples before and after Pan Retinal Photocoagulation Recently, a multicenter clinical trial by "the Central Vein Occlusion Study" (CVOS) group investigated the role of panretinal photocoagulation in ischemic CRVO.4 The purpose of that study was twofold: (1) to find out whether prophylactic panretinal photocoagulation prevents development of iris and angle neovascularization, and (2) whether panretinal photocoagulation prevents progression of iris/angle neovascularization to neovascular glaucoma.
The most important feature of any study is its design, because that can determine its conclusions and their validity. Based on my clinical and experimental study on CRVO during the past 30 years, I have some important concerns about the baseline design of the study[25]. It is now well-accepted that neovascular glaucoma is a complication only of ischemic CRVO and is NOT seen in non-ischemic CRVO. For a study claiming beneficial effects of panretinal photocoagulation on anterior segment neovascularization in ischemic CRVO, it is imperative to ask at least the following three very basic questions:
When all these facts are put together, a very different perspective on ischemic CRVO emerges - and, consequently, a different perspective on its management. In ischemic CRVO, if the eyes either do not develop neovascular glaucoma (and at least 50% will not - Figure 15) or their high IOP due to neovascular glaucoma is controlled satisfactorily by the means discussed below, once the retinopathy burns itself out, they are frequently left with a large, dense, absolute central scotoma (from permanent macular damage invariably seen in this disease), but with a normal or a reasonably good peripheral visual field. The latter is very useful for "getting around" in daily life. By contrast, in ischemic CRVO eyes treated with panretinal photocoagulation, the eye is left with extremely limited visual capabilities, due to a combination of large absolute central scotoma with marked visual field constriction. Therefore, panretinal photocoagulation does more harm than good to the majority of these eyes (Figures 16,17); it neither confers a statistically significant protection against neovascular glaucoma, nor always prevents development of ocular neovascularization, nor confers any other significant benefit. This is particularly undesirable in the 50% or more of ischemic CRVO eyes which would never have developed neovascular glaucoma anyway (Figure 15) and thus did not need panretinal photocoagulation. The high risk of marked peripheral visual field loss from panretinal photocoagulation combined with the naturally developing central scotoma from the disease itself (Figures 16,17), is a serious disabling complication of panretinal photocoagulation without any countervailing benefit.
As is evident from the discussion above, there is no scientifically valid proof so far that panretinal photocoagulation is safe and effective in prevention or management of neovascular glaucoma in acute ischemic CRVO. In spite of that and ignoring the fact that panretinal photocoagulation is highly destructive to the remaining peripheral visual fields in most of the acute ischemic CRVO cases (Figures 16,17), there are authors who still actively advocate its use[4,28].
Since neovascular glaucoma is the most dreaded complication of ischemic CRVO, naturally the question arises how to manage ischemic CRVO. For a logical management of any disease, first one has to understand the basic issues involved and the information available which should act as guidelines. In ischemic CRVO, we have currently the following information available:




I find no convincing scientific evidence that panretinal photocoagulation usually helps prevent development of neovascular glaucoma, in spite of claims made to that effect[4].
In the light of these facts, I follow the following regimen of management of these patients:
With this treatment regimen, I have been able to tide many of these eyes over through the first 7-8 months, or until the retinopathy starts to resolve and the stimulus for anterior segment neovascularization starts to subside. After that these eyes start to settle down. So long as the intraocular pressure is maintained within reasonable limits, the eyes maintain the residual peripheral vision. However, a few eyes very rapidly go into fulminant neovascular glaucoma and no amount of any treatment can control the intraocular pressure. In our studies on panretinal photocoagulation, we saw some eyes develop fulminant neovascular glaucoma in spite of early and extensive panretinal photocoagulation of up to about 3,500 burns and finally become totally blind and even developed phthisis bulbi.
Thus, the prevailing impression among ophthalmologists that ischemic CRVO always has a very bleak prognosis and is always associated with neovascular glaucoma and total blindness is shown to be not true for a majority of the eyes in our studies. With proper management and perseverance, many of these patients can maintain a good peripheral vision which is very helpful in steering themselves in this world. In contrast to that, if panretinal photocoagulation is done in every eye with acute ischemic CRVO with the hope of preventing neovascular glaucoma, as is often advocated, then a vast majority of the eyes are going to lose their peripheral vision and that combined with a large central scotoma is going to convert most of the eyes practically blind which otherwise would have had good peripheral vision - that is not a good medicine. Of course, I have seen eyes which became totally blind and even developed phthisis bulbi no matter what treatment was given but those are in a small minority.
Retinal and optic disc neovascularization without neovascular glaucoma tend to develop late (Figure 15), usually when the retinal edema and hemorrhages have largely resolved. In those cases, I do advocate doing panretinal photocoagulation at this late stage in the evolution of the retinopathy; this is because the risk of panretinal photocoagulation causing severe peripheral visual loss is much less at this late stage than during the acute phase, when there is marked retinal edema and hemorrhages. The reason is that much higher laser intensity is required to produce a satisfactory laser burn when the retina has marked edema than when retina has little or no edema. Also, marked retinal hemorrhages during the acute phase of retinopathy absorb the heat. Thus, a combination of these two factors during the acute stages of retinopathy produces marked retinal damage and marked loss of peripheral visual field due to panretinal photocoagulation.
It is well-known that "a disease which has no treatment has many treatments" – each advocated enthusiastically for a while and then found to be not only no better than the natural history of the disease but also inadequate or even harmful. Most important fact is that a treatment which does not have a sound science to backup does not work in the long run, howsoever aggressively it may be advocated. CRVO has become a graveyard of such therapies. It is unfortunate when such claims give desperate patients false hope, cost them so much, not only in money but also in trouble, unnecessary pain and disappointment, and sometimes do them more harm than good. In this and my other recent review6, I am aware, I have challenged some of the conventional wisdom in the management of CRVO. That is never popular. To advocate "watchful waiting" rather than action is even more unpopular. I can only hope that science, logic and common sense will prevail in the end.
Our data on CRVO, giving full, scientifically based information on these questions, can be found elsewhere [3]. Very briefly, following are some answers:
Document History: Initially posted 1996, Revised July 1999, Revised December 2000; revised and expanded March 2003, expanded July 2010, reviewed March 7, 2014 © text and images, 2003, Sohan Singh Hayreh. Reproduction of any part of this material is not permitted without express permission from Dr. Hayreh.