Chief Complaint: Left-sided periorbital swelling.
History of Present Illness: A 16-month-old male patient presents to the UIHC Oculoplastics Clinic for evaluation of left-sided periorbital swelling. The swelling began three weeks prior to presentation in the setting of an upper respiratory infection. A short course of diphenhydramine did not relieve his symptoms. When he did not respond to a ten-day course of amoxicillin for presumed preseptal cellulitis, he was admitted to an outside hospital for IV antibiotic therapy. He did not improve with this more aggressive therapy and was transferred to UIHC for further workup and management.
He has no other systemic illnesses and has been behaving normally without changes in appetite, sleep patterns, or energy level since the onset of the periorbital swelling.
PMH: The patient was born full-term after an uncomplicated pregnancy. He had reached all of his developmental milestones and was growing normally. His immunizations are up to date.
Meds: None
Allergies: None
FH: No significant family history.
SH: The patient lives with his parents, who are both present for the appointment.
VA: Fixes and follows OD, Fixes and follows OS.
Pupils: No relative afferent pupillary defect.
Motility: Full OU.
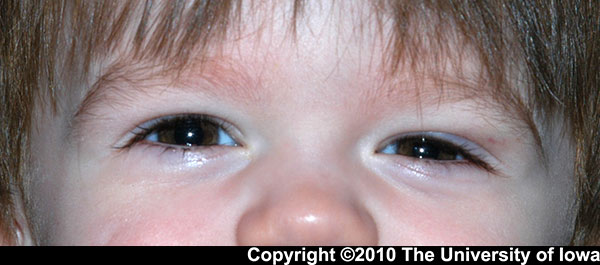
External exam: Fullness and erythema of temporal aspect of left upper eyelid (see Figure 1).
Anterior segment exam: Clear conjunctiva OU, clear corneas OU, deep and quiet anterior chambers OU, normal irides OU, clear lenses OU.
 |
A CT scan done at an outside facility as part of his workup revealed a small, well-defined osteolytic lesion (11.3 x 10.9 x 9.6 mm) in the superotemporal left orbit, eroding into the adjacent frontal bone (See Figure 2).
An MRI was obtained when the patient arrived at UIHC as part of his inpatient workup and corroborated the CT findings (Figure 3 and 4).
The location and osteolytic characteristics of this orbital lesion were highly suggestive of an orbital eosinophilic granuloma. The patient's parents consented to an orbitotomy and biopsy of the lesion for definitive diagnosis.
An uncomplicated left anterior orbitotomy through a superior lid crease incision was performed later that week. The mass was identified just underneath the orbital rim and a biopsy was obtained. Intraoperative frozen sections demonstrated the lesion to be predominantly composed of histiocytes.
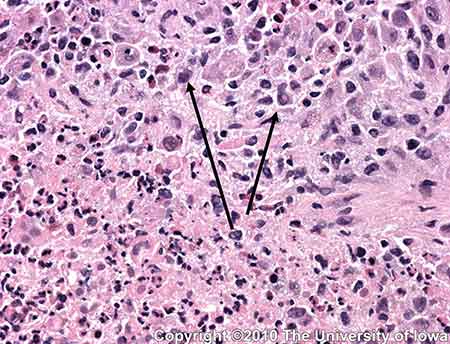
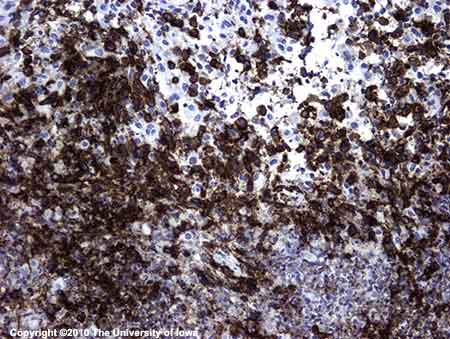
Permanent sections confirmed the presence of a histiocyte-rich inflammatory infiltrate also with some eosinophils, neutrophils and focal areas of necrosis. The histiocytes demonstrated nuclear atypia (irregular nuclear contours with prominent nucleoli) (Figures 5 and 6). Immunohistochemistry with CD1a (specific for histiocytes) was positive (Figure 6). These findings are consistent with the diagnosis of eosinophilic granuloma.
 |
 |
The diagnosis of orbital eosinophilic granuloma was communicated to the patient's parents and pediatric oncology team. A bone scan was performed to evaluate for disseminated disease and was negative. Diabetes insipidus was also ruled out with a concentrated first morning void.
The patient was followed by a pediatric hematology oncology team, who felt that the orbital location of the lesion conferred a higher risk of eventual CNS involvement. It was therefore decided to treat the patient with systemic vinblastine for six weeks and oral prednisone for one month (with taper) and to reassess after this course. Thus far, the patient has tolerated his treatments well.
Eosinophilic granuloma is one disease entity in the Langerhans cell histiocytosis (LCH) spectrum of disorders. LCH represents a group of rare disorders characterized by abnormal histiocytic proliferation. Previously, it was thought that LCH was comprised of three distinct subtypes: solitary eosinophilic granuloma, Hand-Schuller-Christian disease (multifocal lytic lesions) and Letterer-Siwe disease (fatal disseminated disease). More recently, LCH disorders have been re-conceptualized as multiple disease entities that lie along a spectrum from unifocal disease (as in our patient) to disseminated disease (Vosoghi, 2009). While the LCH diseases are generally considered to be diseases of childhood with the peak age of incidence between the ages of 1 and 4 years, the disease has been reported in adulthood as well (Cheung, 2007). Most studies report that males are affected more frequently than females.
Normal Langerhans cells are involved in immunosurveillance as they engage and present antigens to T-cells. The proliferation in the LCH disorders is thought to be clonal, but the inciting event that results in the abnormal proliferation is not known. The proliferating cells produce abnormal levels of interleukin-1, a major osteoclast-activating factor and inhibitor of bone formation, thereby producing the classic osteolytic lesion of LCH (Harris, 2003). The histiocytic proliferation happens preferentially in areas of hematopoietically active bone marrow, which explains the predilection for the superotemporal quadrant of the orbit in orbital disease. The marrow of the maxilla and zygomatic bones lose their hematopoietic activity in infancy, but the frontal bone remains hematopoietically active thorough adulthood (Harris, 2003). The greater sphenoid wing also contains marrow and is a less common site of unifocal disease.
Histologically, the lesions demonstrate histiocytic proliferation accompanied by eosinophils. Nuclear atypia is often seen in the histiocytes. The histiocytes are positive with immunocytochemical stains CD1a and S-100. Electron microscopy often demonstrates the pathognomonic Birbeck granules, cytoplasmic inclusions that are present in about 50% of cases (Henderson, 2007).
Clinical manifestations
The solitary orbital eosinophilic granuloma occupies the benign end of the LCH spectrum. It usually represents unifocal disease without disseminated lytic lesions or other systemic complications. The lesion is usually located in the superotemporal quadrant of the orbit and most commonly results in an osteolytic defect of the orbital roof. Patients often present with progressive upper eyelid edema and erythema with proptosis and localized tenderness. Patients may be unsuccessfully treated for infection or allergy before the diagnosis of eosinophilic granuloma is correctly made.
Workup
CT imaging of these patients best demonstrates the bony erosion caused by these lesions. In early stages, the solitary eosinophilic granuloma appears as a radiolucent area adjacent to bone. As the soft tissue component breaks through the bony cortex, moth-eaten osteolytic defects can be appreciated, as in the case of our patient (Henderson, 2007).
All patients with eosinophilic granuloma of the orbit must be referred to a pediatric oncologist for evaluation of systemic disease. Patients typically undergo a bone scan to rule out multifocal osteolytic lesions. If this is negative, a bone marrow biopsy is not indicated. As diabetes insipidus can be a feature of systemic involvement, patients are screened for this as well.
Treatment
Solitary eosinophilic granuloma of the orbit has been reported to respond well to local treatment alone. A common approach to isolated orbital disease includes biopsy for diagnosis along with total or sub-total curettage of the lesion, with or without the injection of intralesional steroids. The bony defect typically re-ossifies, and patients are then followed closely for recurrence or reactivation.
There is evidence to suggest that patients with craniofacial lesions (including orbit, oral cavity and auricular region) are at a higher risk of developing diabetes insipidus. One proposed mechanism is that the granulomatous process can extend intracranially through perivascular extension to the hypothalamic-pituitary region and result in diabetes insipidus (Grois, 2006). Given this, some propose treating isolated orbital lesions with systemic chemotherapy and systemic steroids. However, the designation of solitary orbital lesions as "CNS risk lesions" is controversial, especially in light of the numerous cases of isolated orbital disease that respond to local therapy alone and do not progress to CNS involvement (Harris, 2004). In spite of this controversy, most can agree on the need for close surveillance with aggressive therapy administered to patients who demonstrate recurrence or progression to multifocal disease.
EPIDEMIOLOGY
|
SIGNS
|
SYMPTOMS
|
TREATMENT
|
Cheung N et al. Orbital Langerhans Cell Histiocytosis in Adults. Ophthalmology. 2007; 114(8):1569-1573.
Grois N et al. Risk Factors for Diabetes Insipidus in Langerhans Cell Histiocytosis. Pediatr Blood Cancer. 2006; 46:228-233.
Harris G et al. Eosinophilic Granuloma of the Orbit: A paradox of aggressive destruction responsive to minimal intervention. Trans Am Ophthalmol Soc. 2003; 101:93-105.
Harris G. Letter to the Editor: Is Unifocal Langerhans-Cell Histiocytosis of the Orbit a CNS-Risk Lesion Pediatr Blood Cancer. 2004; 43 298-299.
Harris, G et al. Langerhans Cell Histiocytosis of the Orbit: A need for Interdisciplinary Dialogue. American Journal of Ophthalmology. 2006; 141(2) 374-378.
Henderson J and Garrity J. Histiocytic Disorders in Henderson's Orbital Tumors. Mayo Foundation for Medical Education and Research, 2007:267-270.
Vosoghi, H et al. Orbital Involvement in Langerhans Cell Histiocytosis. Ophthal Plast Reconstr Surg. 2003; 25(6):430-433..
Gandhi N, Syed NA, Allen RC: Orbital Eosinophilic Granuloma. Eyerounds.org. September 16, 2010; Available from: http://www.EyeRounds.org/cases/120-orbital-eosinophilic-granuloma.htm.

Ophthalmic Atlas Images by EyeRounds.org, The University of Iowa are licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License.