Chief complaint: Abnormal head position
History of Present Illness: A 79-year-old man initially presented to the oculoplastics clinic for treatment of a chalazion on the left eye and follow-up care of his right anophthalmic socket. The patient’s wife pointed out a right head turn when the patient was watching television, which she thought had progressed over the past several years.
Past Medical History: Osteoarthritis, hypertension, hyperlipidemia, atrial fibrillation, depression
Medications: Alprazolam, artificial tears in the left eye as needed, ascorbic acid, celecoxib, cholecalciferol, hydrocodone/aspirin, metoprolol, prednisolone 1 drop in the left eye daily, rosuvastatin, sertraline, tramadol, warfarin
Allergies: No known drug allergies.
Family History: Patient’s son has glaucoma.
Social History: Patient has a history of prior tobacco use, occasional alcohol use.
Review of Systems: Pertinent findings are in the HPI.
OD: Anophthalmia
OS: 20/50-3 pinhole 20/40-2
Pupil: Briskly reactive to light OS
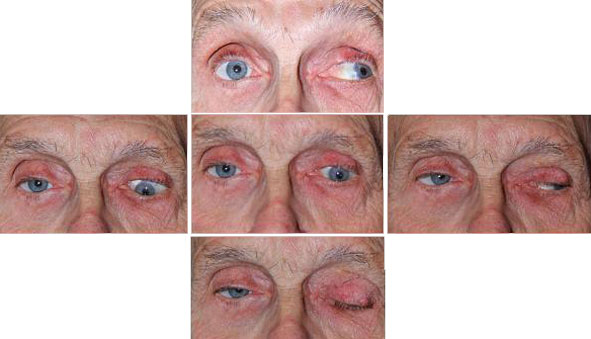 |
External: OD – prosthesis with fat atrophy. OS – fat atrophy.
Dilated Fundus Exam OS: Disc with cup-to-disc ratio of 0.5. Normal macula, vasculature and periphery.
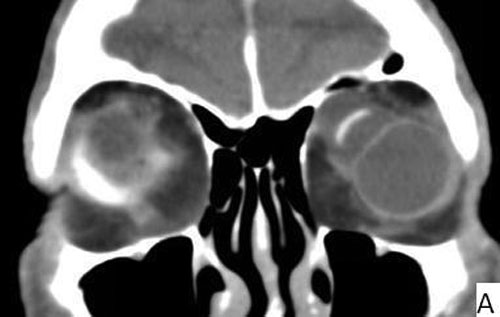 |
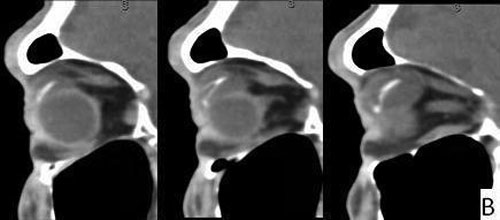 |
| 3A: Coronal view shows large seton reservoir with endplate visible as a hyperintense linear band superonasal to the globe surrounded by cystic area | 3B: Sagittal views of the left orbit |
Diagnosis: Giant reservoir formation from Ahmed seton valve
When Ahmed seton valves are implanted to help control intraocular pressure in glaucoma, the aqueous humor drains through a unidirectional valve and is then resorbed through intercellular spaces into the adjacent periocular tissues. The device is made up of three parts: (1) a drainage tube made of silicone which is placed in the anterior chamber; (2) a unidirectional valve designed for resistance to aqueous outflow; and (3) an endplate available in three various sizes which sits on the exterior surface of the globe. Complications of Ahmed valves include tube blockage, tube exposure, hypotony and encapsulated bleb formation. In bleb encapsulation, the Tenon’s capsule adheres to the episcleral space, causing the bleb to become impervious to aqueous humor. If this occurs, the intraocular pressure can increase, and the bleb can become unusually large, forming a giant reservoir. With time, the bleb can become less dense, leading to normalization of intraocular pressure. In the current case, the patient experience hypotony after his Ahmed seton placement, after which is pressure normalized and remained well-controlled, without a period of increased intraocular pressure.
Encapsulation has been found to be more common in Ahmed valves than other glaucoma drainage implants, which is postulated to be driven by a fibrotic response stimulated by inflammatory factors. In an Ahmed valve which is designed for immediate aqueous filtration, these inflammatory factors may provoke a stronger fibrotic response in the subconjunctival space as compared to a ligated, non-valved implant (Tsai, 2003). The rate of bleb encapsulation has also been reported to be related to differences in biomaterial and shape of the endplate (Ayyala, 2000).
Jeon at al. (Jeon, 2007) reported the case of a 22 year old man with progressive proptosis and pain in his right eye lasting one week. The patient had a history of blunt trauma at age 14, neccesitating a penetrating keratoplasty OD. He developed secondary glaucoma six years later, which was refractory to medical treatment, and an Ahmed model FP-7 implant was placed two years prior to onset of proptosis. The patient’s intraocular pressure had increased from a baseline of 21 mmHg to 28-30 mmHg. An MRI of the orbits was obtained, which showed a large fluid collection surrounding the endplate of the implant, described as a thin-walled, cystic lesion partitioned by a centrally located curvilinear band representing the silicone endplate.
Danesh-Meyer and Spaeth (Danesh-Meyer, 2002) reported a similar case of a 16 year old man with secondary glaucoma after trauma, who presented with progressive proptosis and inferior globe displacement which developed two months after an Ahmed tube shunt was placed. In this case, the patient’s intraocular pressure was 21 mmHg. He had significant limitation of elevation and abduction and exposure keratitis OD. In addition he was unhappy with the cosmetic appearance. MRI imaging demonstrated the mass effect of the giant reservoir on the right eye. The patient was followed conservatively, and his intraocular pressure remained stable. He had no diplopia due to poor vision in the right eye. At 6 week follow-up, proptosis had decreased, measuring a 1 mm difference on Hertel’s exophthalmometry measurements, a 5 mm decrease from the patient’s initial visit. There remained only a mild limitation of elevation OD. At 1 year follow-up, the appearance was deemed cosmetically tolerable, and no intervention was necessary.
Coats et al. (Coats 1999) described an acquired Pseudo-Brown’s syndrome after an Ahmed valve was placed superonasally OS. Strabismus surgery did not correct the problem, but forced ductions necessitated valve repositioning, which improved the patient’s symptoms. Ball et al.6 reported a Brown’s syndrome from a Baerveldt placed superonasally, in which the patient’s symptoms of limitation in elevation with adduction became exaggerated by laser suture lysis, resulting in aqueous expansion. In this case, the patient was ultimately taken for decompression of the encapsulation, which alone moderately improved intra-operative forced ductions, and implant removal, with which the patient’s symptoms resolved.
Eibschitz-Tsimhoni et al. (Eibschitz-Tsimhoni, 2005) studied the incidence and management of encapsulated cysts after the placement of Ahmed seton valves. The study included 62 consecutively placed Ahmed valve S2 model setons, 57 of which had one year follow-up data. Of this group, 13 patients developed encapsulated cysts, defined as the appearance of a localized, elevated dome-shaped bleb with elevation of intraocular pressure. The patients were all treated with anti-glaucoma medications with or without digital ocular pressure. If medical therapy did not decrease the intraocular pressure to target pressure, the patient underwent transconjunctival needling with 5-fluorouracil injections. If the intraocular pressure was still not at goal, surgical excision of the cyst wall was performed. Of the 13 patients with encapsulated cysts, one responded to medical treatment alone, one developed secondary restrictive strabismus prompting surgical excision, and 11 patients required surgical excision based on not meeting target. Despite this aggressive therapy, the mean intraocular pressure of the group needing surgical intervention was 34.6 mmHg (range 27-47 mmHg). Surgical excision was performed, resulting in successful intraocular pressure control in 8 of 11 patients. The three other patients required additional surgery for recurrence of encapsulation.
Various other studies have reported the rate of Ahmed valve encapsulation ranging from 0% (Wang, 2004) to 25% (Perez, 2000). It is difficult to compare studies to one another however, as not all studies consider encapsulation a post-operative complication and therefore do not report its occurrence.
In the current case, the patient had an Ahmed valve seton FP-7 model, which has a flexible endplate made of silicone, in contrast with the S2 model of the Ahmed valve seton, which is made of polypropylene. The case reported by Jeon et al. (Jeon, 2007) involved a silicone FP-7 model, whereas Danesh-Meier and Spaeth (Danesh-Meyer, 2002), as well as Coats et al. (Coats 1999) did not report the model used. Eibschitz-Tsimhoni et al.’s (Eibschitz-Tsimhoni, 2005) study of 57 consecutively placed Ahmed valve setons used only the S2 model. In a prospective, multicenter comparative series, Ishida et al. (Ishida, 2006) studied 66 patients implanted with a FP-7 model Ahmed valve seton and 66 patients implanted with a S2 model, and found 12 of 66 (18.2%) S2 model patients developed a Tenon’s cyst, as compared with 3 of 66 (4.5%) in the FP-7 cohort (p = 0.026). Brasil et al. (Brasil, 2007) also found a higher rate of plate encapsulation in patients with S2 model Ahmed setons (4/70, 5.7%) versus patients with FP-7 model Ahmed setons (3/110, 2.7%) in their retrospective chart review. The difference between the scar formation of Ahmed valve seton models has been attributed to a more intense inflammatory response to the rigid polypropylene than the flexible silicone endplate, as found by Ayyala et al. (Ayyala, 1999) in rabbit eyes based on conjunctival vascular hyperemia, histology and scanning electron microscopy. The polypropylene endplate of the S2 model Ahmed seton has been shown to have a higher rate of encapsulation than the FP-7 silicone model, although the studies evaluating the difference in complication rates and the case reports noted above show that pathologic encapsulation of the FP-7 endplate also occurs.
Our patient’s case is notable for lack of diplopia due to anophthalmia OD. The motility deficits also directed the differential to include an oculomotor nerve palsy, which prompted CT imaging to confirm the diagnosis and rule out gross intracranial abnormality. Based on the clinical history and exam, B-scan echography alone may be a useful imaging modality. The patient’s wife was concerned about his abnormal head position, which had gradually increased. The patient’s intraocular pressure was well-controlled. The treatment options discussed included strabismus surgery to improve primary gaze alignment and head position, as well as Ahmed seton valve revision. Ultimately, there was a strong preference for following the patient conservatively due to his well-controlled intraocular pressure, prior hypotony and choroidal hemorrhage with Ahmed valve placement initially, lack of diplopia and monocular status.
Diagnosis: Giant reservoir formation from Ahmed seton valve
Summary
|
Signs
|
Symptoms
|
Treatment
|
Kemp PS, Allen RC, Kwon YH. Giant reservoir formation from Ahmed seton valve. Eyerounds.org. June 2, 2012, Available from: https://eyerounds.org/cases/150-Giant-Seton-Reservoir.htm.

Ophthalmic Atlas Images by EyeRounds.org, The University of Iowa are licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License.