Elevated intraocular pressure and "cloudy, hazy" vision of the left eye
A 54 year-old-man was referred for elevated intraocular pressure (IOP) and decreased vision in the left eye (OS). His ocular history was remarkable for cataract extraction with intraocular lens (IOL) placement in both eyes (OU) and retinal detachment OU repaired by pars plana vitrectomy OU. Eight months prior to presentation, the IOL (Acrysof SN60AT single-piece lens) in the patient's left eye became displaced, and was subsequently repositioned in the ciliary sulcus. The patient developed macular edema in his left eye three months later for which he received a sub-Tenon triamcinolone acetonide injection and started prednisolone acetate eye drops. Two months prior to presentation, the patient developed an IOP of 48 mmHg OS. The patient's left eye was treated with latanoprost every night, timolol-brimonidine (Combigan©) two times a day, and dorzolamide two times a day. He also started oral acetazolamide 500 mg two times a day. One week later, IOP was noted to be in the upper teens, and acetazolamide was discontinued. However, two weeks prior to presentation, his IOP was again elevated to 52 mmHg, and acetazolamide 500 mg two times a day was restarted. Pigment was noted in the anterior chamber one week prior to presentation, and he was restarted on prednisolone acetate eye drops. The patient was referred to the Department of Ophthalmology at the University of Iowa Hospitals and Clinics for evaluation.
Traumatic hyphema of right eye (OD) secondary to a bungee cord injury (30 years prior)
None
Clindamycin (tooth abscess)
No known drug allergies
Father with glaucoma, retinal detachment
Social alcohol use, no tobacco use
Unremarkable
*Note: A description of the Spaeth grading system is available at http://glaucomatoday.com/pdfs/0505_0506.pdf
 |
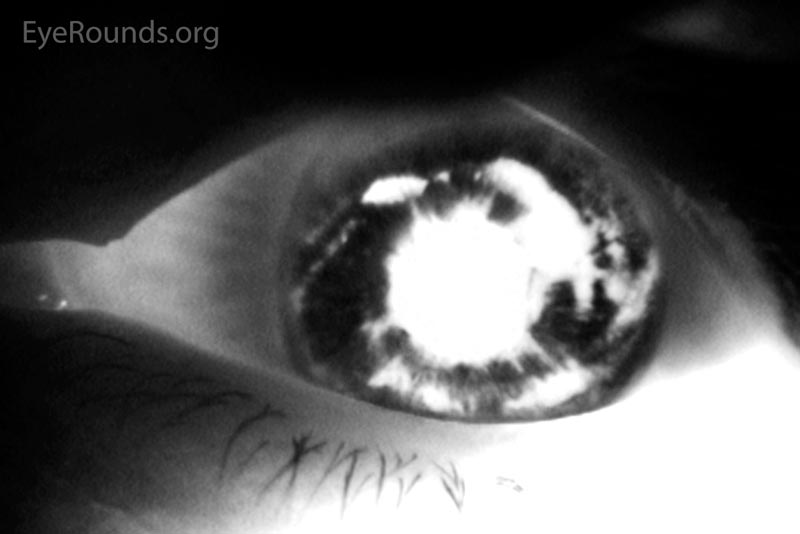 |
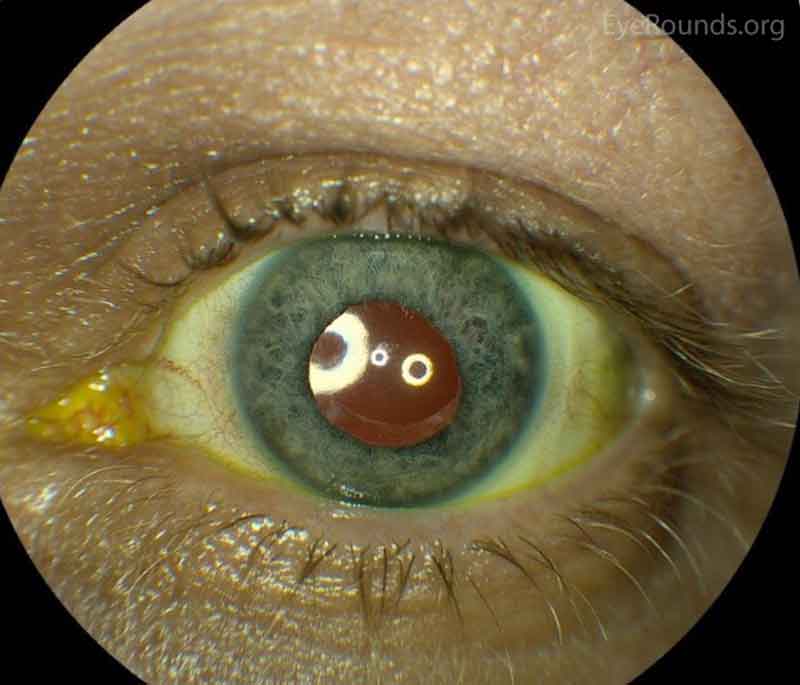
Figure 1: Color and infrared transillumination photos of the left eye. Multiple radial transillumination defects of the iris as well as circumferential defects that match the shape of the IOL haptics.
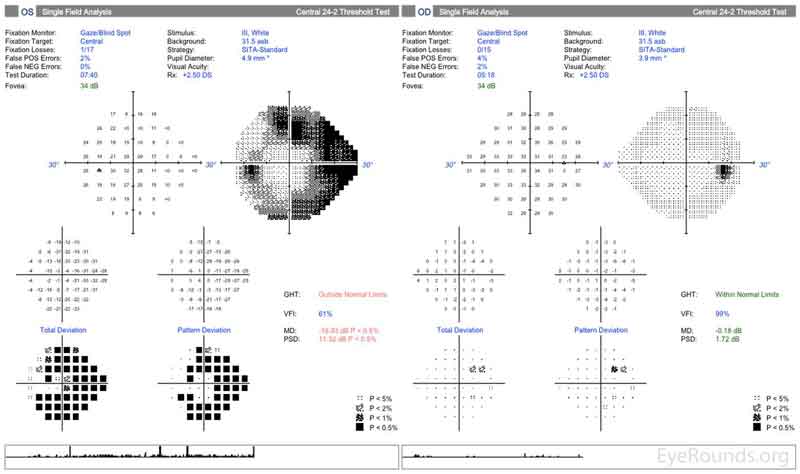
Figure 2: Humphrey Visual Fields (24-2). Left image: Normal visual field OD with good test reliability. Right image: Good test reliability with evidence of severe stage glaucoma with superior and inferior arcuate scotomas OS.
The patient underwent a pars plana vitrectomy (23 gauge) with removal of the single-piece IOL and placement of a 3-piece sulcus IOL (Acrysof MA60AC, Alcon Laboratories, Ft. Worth, TX). Immediately following the PPV, an Ahmed seton (model FP7, New Word Medical, Rancho Cucamonga, CA) was inserted. By post-operative week 16, the visual acuity was 20/25, and IOP was 14 mm Hg on timolol-brimonidine (Combigan©) two times daily.
Uveitis-Glaucoma-Hyphema-Syndrome
Uveitis-glaucoma-hyphema (UGH) syndrome was first described by Ellingson in 1978 and classically included uveitis, glaucoma, and hyphema in the setting of an anterior chamber IOL.[1] However, the term UGH syndrome is often used when one, two, or all three of these entities are present in the setting of any IOL causing irritation of the iris or angle structures.
Although the incidence is not known, UGH syndrome is thought to be a rare condition that can occur in any patient population, including pediatric populations.[2] UGH syndrome was first associated, and is more commonly associated, with anterior chamber IOLs, single-piece acrylic IOLs, or sulcus lenses;[3] however, it can be seen with any type of IOL, including posterior chamber lenses and cosmetic iris implants.[4] With the predominant use of posterior chamber IOLs and modern advances in lens design, the incidence of UGH syndrome has declined.
UGH syndrome results from an IOL chafing the iris, iridocorneal angle, or ciliary body, which leads to recurrent trauma to these structures. Uveitis results from mechanical breakdown of the blood aqueous barrier and resultant inflammation. A hyphema results from recurrent damage by the IOL to vascular tissue of the iris, ciliary body, or angle. Intraocular pressure elevation can be caused by pigment dispersion, uveitis, hyphema, direct injury to the aqueous drainage system, or a steroid response to corticosteroids used to treat UGH-related inflammation. Given that IOL irritation underlies the etiology of UGH syndrome, selection of the appropriate lens type, design, and size is crucial to minimize the risk of developing UGH syndrome.
Anterior chamber IOLs have traditionally been associated with UGH syndrome, as they were used when the first cases of UGH syndrome were described. The sizing of an anterior chamber IOL is crucial and should be approximated by measuring the horizontal white-to-white corneal diameter (limbus to limbus) and adding 1 mm. The anterior chamber IOL size and positioning should be assessed following implantation to ensure optimal fit. A small anterior chamber IOL may rotate or tilt to contact iris, whereas a large anterior chamber IOL may directly contact and irritate the iris and angle structures to cause UGH syndrome.
Sulcus IOLs are thought to be a more common cause of UGH syndrome given the close proximity to uveal tissue when intentionally or unintentionally placed in the sulcus. IOLs placed in the sulcus with square optic edges and large, thick, square haptics (e.g., single-piece acrylic IOLs) may contact and irritate iris anteriorly to cause UGH syndrome. In addition, sulcus IOLs with small, planar haptics (e.g., Acrysof SN60AT) may more easily dislocate to contact the uveal tissue.[3] An IOL with a thin, posteriorly angulated haptic and a smooth optic surface with rounded edges would be preferable to minimize iris chafing and the occurrence of UGH syndrome. As with anterior chamber IOLs, proper sizing and positioning of sulcus IOLs are crucial to decrease the contact with uveal tissue and chances of UGH syndrome. It has been recommended that single-piece acrylic IOLs and other IOLs designed for the capsular bag not be placed in the sulcus.[2] However, when placed in the sulcus, IOLs should be secure and clear of the posterior iris surface. UGH syndrome may also occur with sulcus IOLs in the setting of reverse pupillary block, especially in susceptible eyes (i.e., myopia, post-vitrectomy).[5]
Posterior chamber IOLs may or may not be completely positioned in the capsular bag to contact uveal tissue and, though more rare, cause UGH syndrome.[6] In the setting of weakened zonules, such as in exfoliation syndrome or IOL subluxation, UGH syndrome is more likely to occur due to poor zonular support and posterior chamber IOL movement.
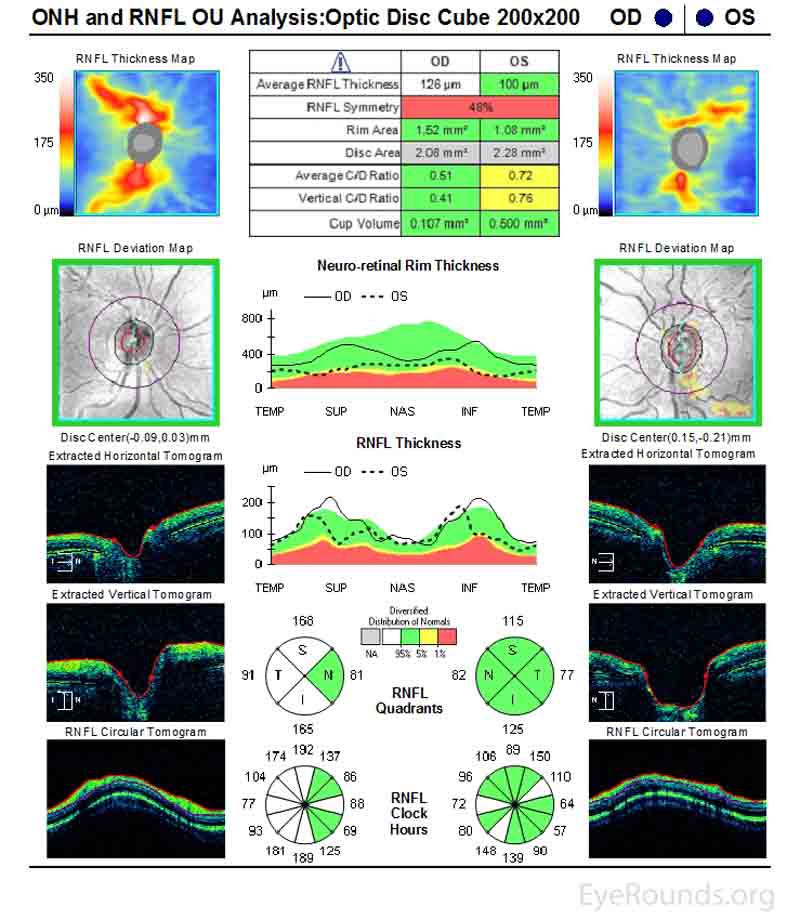
Patients may complain of blurred vision, transient vision loss, ocular pain or ache, erythropsia (i.e., objects take on a reddish hue), or photophobia. Slit lamp examination is essential to help establish the diagnosis. A poorly positioned IOL optic or haptic contacting uveal tissue may be directly observed. Hyphema, cell and/or flare, transillumination iris defects, synechiae, pseudophacodonesis, or corneal pigment may also be identified on the slit lamp examination. Gonioscopy may demonstrate blood within the inferior angle, signs of mechanical erosion, poorly positioned haptics, or increased pigmentation of the trabecular meshwork. Ocular hypertension is often present, and optic disc cupping and glaucomatous vision loss may be present in advanced cases (Figures 2 and 3).
Prothrombin time and international normalized ratio should be tested in patients on anticoagulation medications as these patients may have an increased risk of bleeding.
Ultrasound biomicroscopy and OCT of the anterior segment may be useful to identify IOL position and contact with the iris or angle structures (Figure 4). Posterior segment ultrasound (B-scan) may be useful to identify a vitreous hemorrhage, if present. UGH plus syndrome is the name given to UGH syndrome in the presence of vitreous hemorrhage.
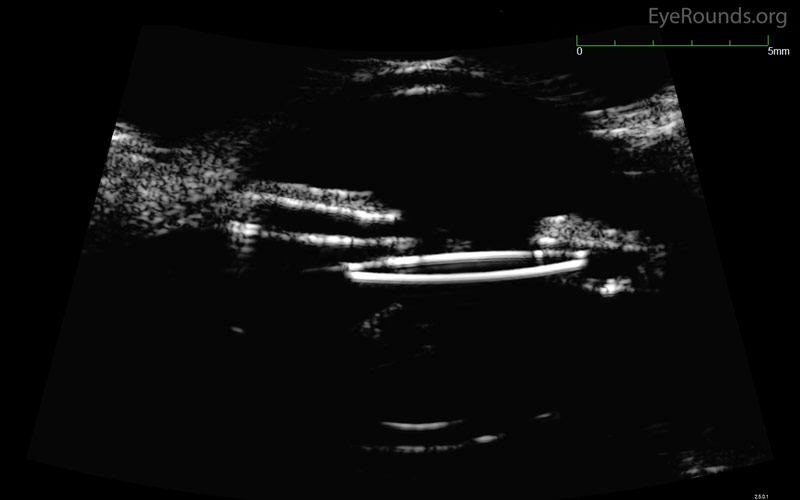
Figure 4: Ultrasound biomicroscopy of the left eye. The nasal optic of an IOL is seen abutting the posterior iris surface in a patient with UGH syndrome.
(Note: Figure 4 was obtained from a different patient and is used in this case for illustration.)
Uveitis: Topical corticosteroid drops
Glaucoma: Prostaglandin analogs, beta-blockers, and carbonic anhydrase inhibitors.
Pilocarpine and other cholinergics should be avoided to prevent further chafing of the iris.
Hyphema: Topical corticosteroids drops and cycloplegics (e.g., cyclopentolate)
Definitive treatment is to secure or exchange the IOL or iris implant. The UGH syndrome is one of the more common indications for IOL exchange surgery, representing 11.9% of IOL exchanges in one study.[7] Laser peripheral iridotomy may be used for the management of UGH syndrome resulting from reverse pupillary block.[5] In addition, serial intracameral anti-VEGF injections have been used to successfully manage UGH syndrome.[8]
EPIDEMIOLOGY OR ETIOLOGY
|
SIGNS
|
SYMPTOMS
|
TREATMENT/MANAGEMENT
|
Cheng L, Fox AR, Kam JP, Alward WLM. Uveitis Glaucoma Hyphema (UGH) Syndrome. EyeRounds.org. posted October 3, 2017; Available from: https://eyerounds.org/cases/257-UGH-syndrome.htm

Ophthalmic Atlas Images by EyeRounds.org, The University of Iowa are licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License.