INITIAL PRESENTATION
Chief Complaint: "I was told the pressure in my eyes is way too high!"
History of Present Illness:
A 37-year-old woman presented as a referral from her local eye doctor who found that her intraocular pressure (IOP) was high on routine eye examination. At that time, her IOP was in the mid-30's in both eyes. She was started on bimatoprost once at night in both eyes and sent to the University of Iowa for further evaluation and management. She denied any vision changes in either eye. She also denied headache or eye pain, and had no history of eye redness, photophobia, glare, or haloes. She did have a history of intermittent itching in both eyes and had been diagnosed with allergic conjunctivitis in the past.
Past Ocular History:
Medical History:
Medications:
Allgergies:
Family History:
Social History:
Review of Systems:
OCULAR EXAMINATION
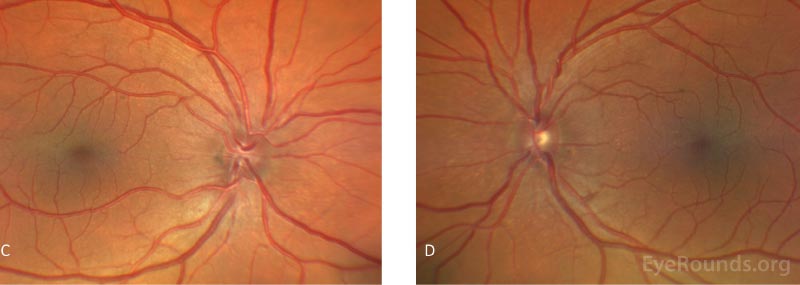
Humphrey visual field tests and Cirrus optic nerve head and retinal nerve fiber layer optical coherence tomography were obtained at initial presentation and were unremarkable.
Biometry was obtained and revealed an axial eye length of 16.93 mm and 16.92 mm in the OD and OS, respectively. Ultrasound biomicroscopy (UBM) showed thickened anterior sclera, no anterior choroidal effusions, and a normal ciliary body as demonstrated in Figure 3. An example of posterior scleral thickening seen in the setting of nanophthalmos can be found here.
Differential Diagnosis:
There is a broad differential for anatomically narrow angles. Below is a brief differential diagnosis for narrow angle, which does not include secondary angle closure that occurs as a result of iridocorneal pathology (e.g. neovascularization, PAS, etc.).
CLINICAL COURSE
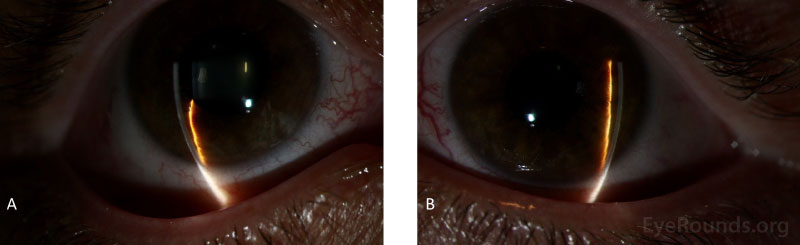
During the initial clinic visit, the patient underwent laser peripheral iridotomies (LPIs) in both eyes. Immediately following the LPI, her IOP did not significantly change, confirming that pupillary block had likely not been a major contributor to her elevated IOP. However, on gonioscopy her angle appeared to be less narrow in both eyes. The patient was started on timolol-dorzolamide twice daily and brimonidine twice daily in both eyes. She was also started on ketorolac four times a day in both eyes for one week following the LPI and scheduled to follow up with her local provider. The decision to treat with ketorolac was made per the Steroids After Laser Trabeculoplasty (SALT) Trial, which demonstrated both prednisolone 1% and ketorolac 0.5% are both effective after LPI [1]. At the one week return visit, the LPIs appeared to have interval closure as the iridotomies' patency was not well visualized on exam. Therefore, iridotomies were repeated one and two weeks after initial presentation, respectively. At her four-week follow up visit, the patient was doing well with demonstrably patent iridotomies and excellent IOPs, which were 15 mm Hg by applanation in both eyes, and therefore further surgical intervention was deferred.
DIAGNOSIS: Nanophthalmos with ocular hypertension and angle closure
DISCUSSION
Epidemiology
Nanophthalmos is characterized by a small eye, generally with an axial length between 14 and 20.5 mm in an adult [2]. A nanophthalmic eye is typically normal in shape but associated with a small corneal diameter and shallow anterior chamber with narrow angles. As the size of the lens is normal, there is a high lens-to-eye ratio with the lens accounting for 10-30% of the globe volume, as opposed to ~4% in normal eyes [2]. The exact incidence of nanophthalmos is unknown and there is no clear gender predilection.
Pathophysiology
In regard to the development of glaucoma, the narrow angles associated with high hyperopia may result in elevated IOP. The angle may progressively narrow with age as the lens enlarges to create pupillary block and can result in the development of peripheral anterior synechiae (PAS). Episodes of angle closure may occur spontaneously due to narrow angles but may also be aggravated by uveal effusion syndrome. At a molecular level, the scleral tissue of nanophthalmic eyes has been characterized by elevated fibronectin levels that may be responsible for thicker, relatively impermeable, and more disorganized sclera [2,3]. These alterations of the scleral fibers can increase the risk for uveal effusion syndrome. When this syndrome occurs, the increased volume from the choroidal fluid collections can yield increased posterior segment pressure causing forward rotation of the ciliary body and iris-lens diaphragm and subsequent angle closure [2]. Angle-closure glaucoma develops when elevated IOP or episodes of elevated IOP lead to cupping of the optic nerve, thinning of the retinal nerve fiber layer, and visual field defects.
Nanophthalmos can occur unilaterally or bilaterally and may be sporadic or inherited as part of a non-syndrome or syndromic disorder [4,5]. If inherited, the syndromes have been described in both an autosomal dominant and autosomal recessive manner [2]. Syndromes that manifest with nanophthalmos are exceedingly rare but include nanophthalmos-retinitis pigmentosa-foveoschisis-optic disc drusen syndrome [6], nanophthalmos and autosomal dominant vitreoretinochoroidopathy (ADVIRC) [7-9], oculo-dento-digital dysplasia syndrome [10], Hallermann-Streiff syndrome [11], and Kenny-Caffey syndrome [3, 12, 13]. Five main genetic loci have been linked to nanophthalmos: membrane frizzled-related protein (MFRP) [14], serine protease 56 (PRSS56) [14], transmembrane protein 98 (TMEM98) [15], BEST1(VMD2) [7], and CRB1 [13, 16]. Of these, it is important to know that MFRP, which is a transmembrane protein expressed in the RPE and ciliary body, appears to play a key role as a regulator of ocular size at birth and ocular growth throughout childhood [17].
Symptoms/Signs
Patients with nanophthalmos are usually asymptomatic and may only complain of being visually impaired without their high plus glasses. Similar to our case, patients with elevated intraocular pressure in the setting of long-standing narrow angles may also be asymptomatic, and elevated IOP may only be picked up on routine examination. Cases of acute angle closure with pupillary block may present with eye pain, redness, glare, haloes, and blurry vision.
The base eye exam is normal except that these patients demonstrate high hyperopia, which is evident on cycloplegic or manifest refraction. In childhood, management of the refractive error is critical in order to prevent amblyopia and accommodative esotropia. Rarely, patients can be emmetropic or myopic, particularly if they have experienced a myopic shift from cataract progression. Intraocular pressure may be normal and can also be elevated in the setting of acute or chronic primary angle closure glaucoma.
Slit lamp examination reveals an eye that is normal in shape but unusually small and with a shallow anterior chamber. A small corneal diameter is also possible though not always present. If the nanophthalmos is occurring in the setting of an associated syndrome, enophthalmos and narrow palpebral fissures may also be present [16]. In cases of acute angle closure with pupillary block, there are characteristic findings including conjunctival injection, corneal edema, anterior chamber (AC) inflammation, a shallow AC peripherally more than centrally, iris bombe, and a mid-dilated pupil. Gonioscopy may also reveal occludable angles with possible PAS in the setting of chronic angle closure.
Fundoscopic examination may reveal glaucomatous damage to the optic nerve, however, the remainder of the posterior segment is usually unremarkable. In the absence of glaucomatous damage, the optic disc may also be small and crowded. Rare fundus findings include optic nerve head drusen, foveal hypoplasia, foveoschisis, papillomacular folds, and retinal pigmentary dystrophies [2, 13].
Visual field testing and optical coherence tomography (OCT) of the optic nerves should be performed to evaluate for glaucoma. Biometry reveals an axial eye length (AEL) usually less than 20 mm and a higher than normal lens thickness (LT) to AEL ratio. Biometry is not only important for confirming that the eye is small, but also in planning for potential cataract surgery or clear lens exchange. B-scan ultrasonography reveals a thickened sclera and may also reveal uveal effusions and subsequent serous retinal detachments. The uveal effusions can also present as annular effusions of the ciliary body that can spare the posterior pole.
Treatment/Management
Based on clinical manifestations, the treatment and management of nanophthalmos with ocular hypertension may vary. Medical therapy includes aqueous suppressants: commonly beta-blockers, carbonic anhydrase inhibitors, and α2-adrenergic agonists [18]. Weak miotics should be used with caution as they may contribute to pupillary block [19, 20].
LPI can be performed as a prophylactic measure to prevent the occurrence of pupillary block and potential acute angle closure and chronic angle closure glaucoma. Laser iridoplasty may also be performed to further widen the angle [18].
Unfortunately, medical and laser therapy alone is seldom enough to control IOP in these patients, especially as the lens enlarges. Clear lens exchange or cataract surgery may be helpful in treating elevated IOP by deepening the angle. However, intraocular surgery and filtration procedures in patients with nanophthalmos presents unique challenges and risks. These risks include posterior pressure from high vitreous pressure, choroidal effusion, exudative retinal detachment, and suprachoroidal hemorrhage.
Other prophylactic measures that can be performed during surgery include sclerectomy or sclerotomy to help reduce posterior segment venous pressure and reduce the risk of intraoperative and post-operative choroidal effusion and suprachoroidal hemorrhage. This holds true for any intraocular surgery including trabeculectomy [20], cataract surgery [22], and repairs for retinal detachment. To further minimize the risk of choroidal effusion, measures should be taken to avoid intraoperative and postoperative hypotony. Management of uveal effusion syndrome is most commonly a full-thickness sclerectomy
EPIDEMIOLOGY OR ETIOLOGY
|
SIGNS
|
SYMPTOMS
|
TREATMENT/MANAGEMENT
|
References
Motlagh M, Quist TS, Fox AR, Pouw A. Nanophthalmos with Ocular Hypertension and Angle-Closure. EyeRounds.org. April 8, 2021. Available from https://EyeRounds.org/cases/312-nanophthalmos.htm

Ophthalmic Atlas Images by EyeRounds.org, The University of Iowa are licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License.