INITIAL PRESENTATION
Chief Complaint: Decreased vision in the left eye
History of Present Illness:
A 47-year-old female presented to clinic with decreased visual acuity in her left eye. Two days prior, she experienced a shadow over her superior visual field accompanied by many black floaters both of which remained present at the time of presentation. She denied any associated flashes of light, pain, or associated headache.
Past Ocular History:
Moderate non-proliferative diabetic retinopathy in both eyes
Medical History:
Medications:
Allergies:
Family History:
Both mother and father had diabetes mellitus and coronary artery disease
Social History:
Non-smoker and does not consume alcohol
Review of Systems:
Negative except for what is detailed in the history of present illness
OCULAR EXAMINATION
CLINICAL COURSE
Slit lamp examination revealed a 1x1 mm superficial telangiectasia on the nasal bulbar conjunctiva OD (Figure 1). The bleeding was thought to be likely due to minor ocular surface trauma secondary to eye rubbing around the telangiectasia, resulting in the reported bloody tears. She has underlying dry eye disease and seasonal allergies that may have contributed to irritation and ocular surface fragility. Her baseline blurry vision was thought to be due to her dry eye symptoms.
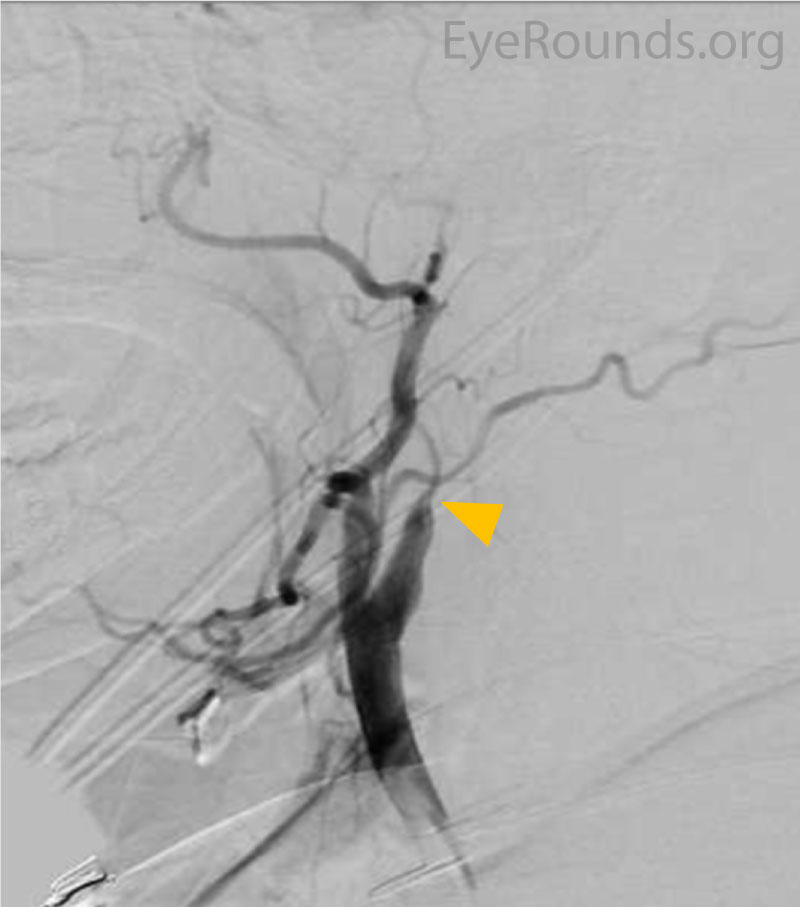
This patient was not a candidate for carotid endarterectomy because her carotid arteries were occluded greater than 99%. She was managed by neurology non-surgically with anti-platelet agents (aspirin and clopidogrel) and discontinuation of blood pressure medications. From an ophthalmic perspective, she was treated with panretinal photocoagulation and received intravitreal bevacizumab injections in both eyes to regress the neovascularization.
Differential diagnosis for the retinal findings of ocular ischemic syndrome:
DIAGNOSIS: Ocular Ischemic Syndrome
DISCUSSION
Epidemiology
Ocular ischemic syndrome (OIS) occurs due to chronic, insufficient perfusion to the eye, typically from severe from internal carotid artery stenosis [1-2]. The annual incidence has been estimated at 7.5 cases per million persons. However, this number is likely an underestimate, as OIS shares similar features with (and may be concomitant with) diabetic retinopathy and central vein occlusion, two far more common retinal vascular conditions that may mask the diagnosis of OIS. The average age at diagnosis for OIS is in the sixth decade of life, and men are diagnosed with OIS at roughly twice the rate of women. Bilateral OIS is found in approximately 20% of patients [3].
Etiology
OIS most commonly occurs secondary to severe, ipsilateral internal carotid artery stenosis leading to chronic hypoperfusion of the eye. Although stenosis is classically in the proximal internal carotid artery, occlusion of more distal segments of the internal carotid artery can also lead to OIS. Ocular perfusion pressure is considered a function of the arterial pressure reaching the eye minus the intraocular pressure (ocular perfusion pressure [OPP] = mean arterial pressure [MAP] – intraocular pressure [IOP]).
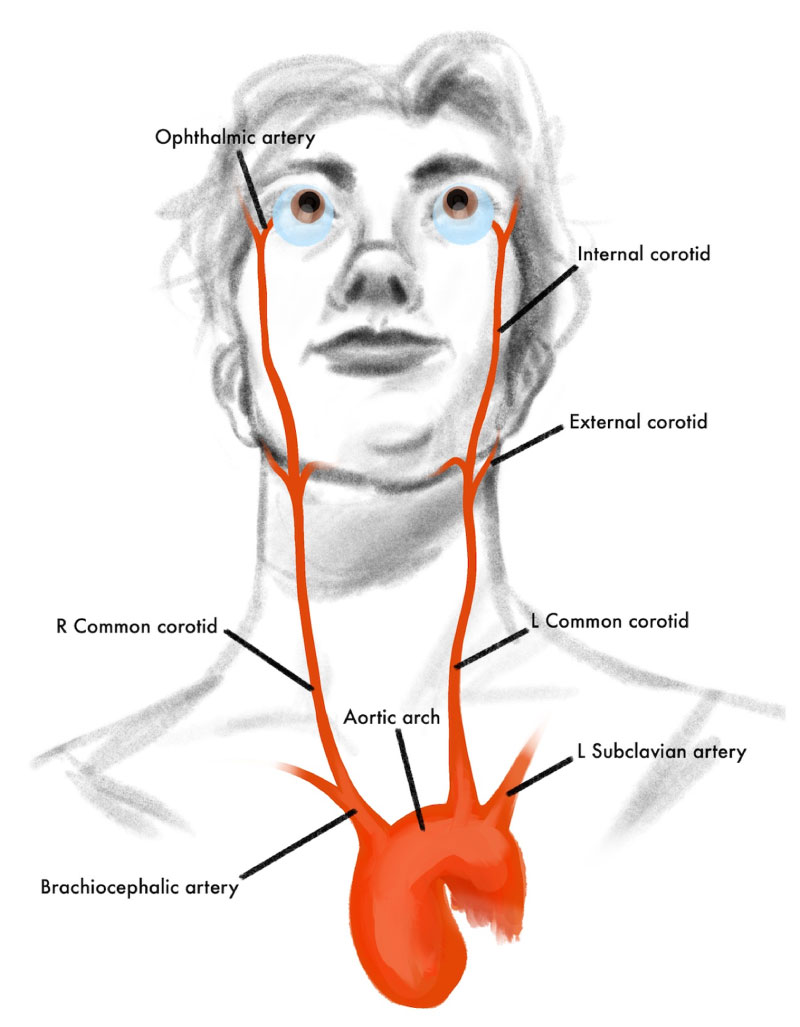
To understand ocular ischemic syndrome, it is important to know the anatomy of blood flow from the heart to the eye. Oxygenated blood exits the left ventricle via the aorta then travels to the common carotid artery, which then bifurcates into internal and external branches (Figure 5). The ophthalmic artery, which supplies the eye and orbit, is the first major branch of the internal carotid artery (Figure 6). In patients with OIS, mean arterial pressure to the eye is decreased, for example, by an obstruction at the level of the proximal internal carotid artery leading to chronically low ocular perfusion pressure and subsequent hypoxic injury to the eye and orbit [1-2].
In most patients, internal carotid artery stenosis is caused by cholesterol plaques secondary to vascular and genetic risk factors [1-2]. In rare cases, internal carotid artery stenosis is secondary to other causes, including inflammatory vasculitis (e.g., from giant cell arteritis) [4-7].
Symptoms and Signs
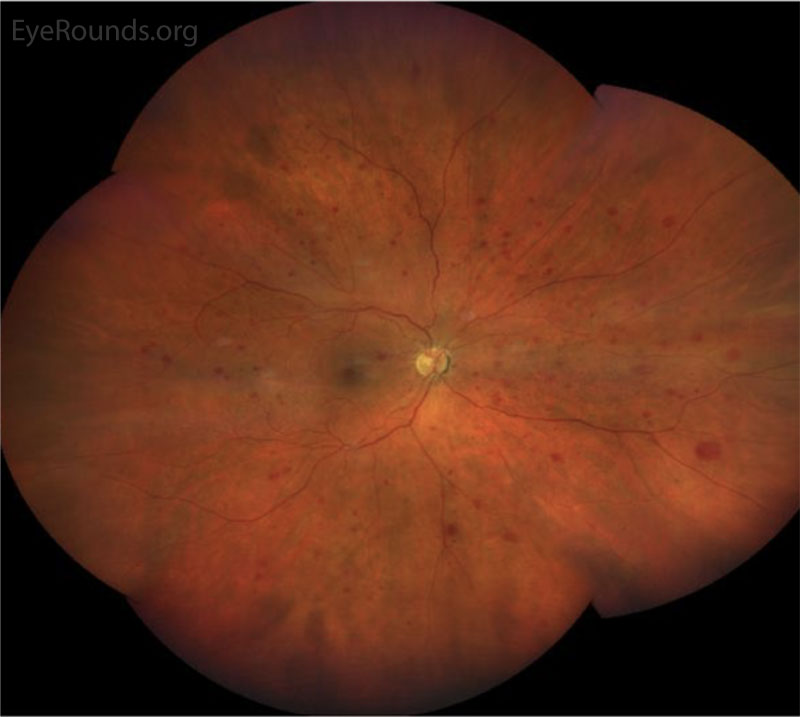
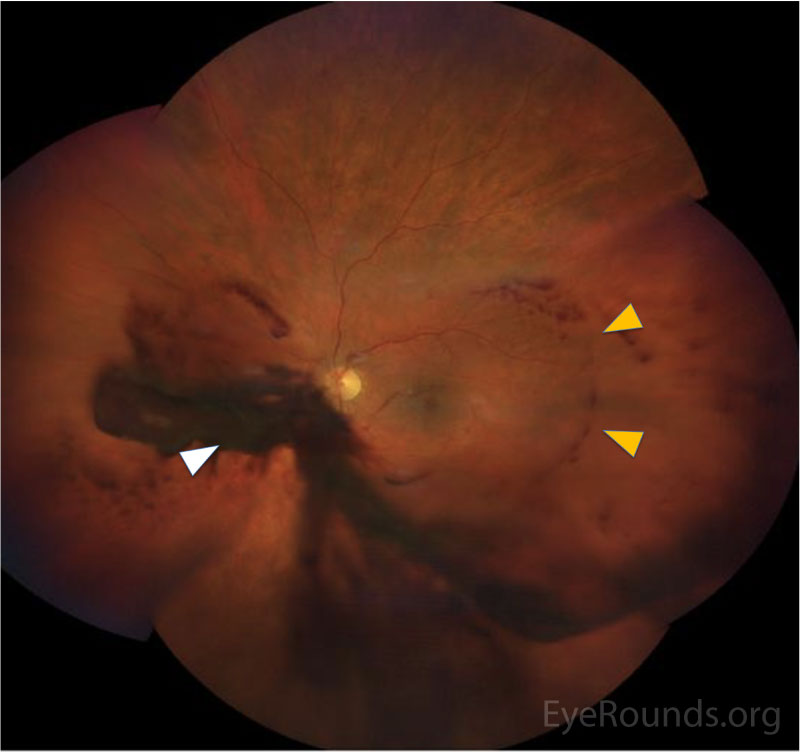
As seen in Figures 5 and 6 above, the ophthalmic artery supplies multiple structures of the orbit and eye. Therefore, chronically decreased perfusion from the ophthalmic artery can lead to pervasive ischemic injury to the orbit and all structures of the eye. Given the heterogeneity of the damage to orbital and ocular structures, the symptoms experienced by patients vary widely. The most common reported symptoms of OIS are vision loss (90%) and eye pain (50%) [1]. However, patients may also report episodes of transient vision loss (i.e., amaurosis fugax), prolonged visual recovery after exposure to light, and red eye [2,8].
In the anterior segment, corneal edema and iris neovascularization may be appreciated on exam. Iris neovascularization is secondary to chronic ischemia causing elevated pro-angiogenic signals, including vascular endothelial growth factor (VEGF), and may led to neovascular glaucoma when neovascularization involves the angle and occludes the trabecular meshwork. Other findings in the anterior segment include cell and flare in the anterior chamber, presumably secondary to reactive inflammation and degradation of anterior segment structures. Progression of cataracts may also be observed [1-2].
In the posterior segment, decreased blood flow to the retina leads to vascular insufficiency and compromise, typically manifested as intraretinal hemorrhages, which classically appear splotchy (i.e., larger than typical dot blot heme seen in other conditions, such as diabetic retinopathy) and in the mid-periphery (versus diabetic retinopathy, where intraretinal hemorrhages may be more concentrated in the macula and posterior pole). Chronic retinal ischemia leads to elevated levels of VEGF and other angiogenic factors, which drives retinal neovascularization and predisposes the patient to vitreous hemorrhages, seen in ~80% of OIS patients. Because carotid atherosclerosis is often a predisposing risk factor, central retinal artery occlusions may also be observed due to emboli from the internal carotid plaque [2].
In addition to the fundus features above, a helpful examination technique for identifying poor ocular perfusion is to apply gentle digital pressure during fundoscopic examination to estimate central artery perfusion pressure (ophthalmodynamometry). In patients with OIS, even light digital pressure on the globe can transiently raise the IOP such that the central retinal artery occludes. It is helpful to compare eyes when using this technique, particularly if the fundus examination of the fellow eye is normal. If a stethoscope is available, it may also be helpful to listen for a carotid bruit.
OIS may present primarily to an eye care provider and should be included in the differential even for patients without confirmed carotid occlusive disease. In previously published studies, most patients (>60%) diagnosed with OIS had no prior carotid artery imaging performed [1]. The 5-year mortality rate for OIS has been calculated to be as high as forty percent due to the high burden of severe atherosclerotic disease associated with OIS. Thus, identification of OIS may be a warning sign of serious systemic disease, and prompt workup and subsequent interventions may reduce the risk of death [9].
Testing/Imaging
If there is concern for OIS in a patient, the next step is to image the carotid arteries to identify a possible stenosis. The first line imaging modality is carotid duplex, but it is important to recognize that distal carotid artery occlusion can be missed. CT angiogram or MR angiogram may also be used and are advantageous because the entire carotid artery is imaged. Cerebral angiogram is helpful in some cases but is more invasive and may not be preferred in some cases due to the risk of stroke. Greater than 90% carotid occlusion is commonly seen in OIS. Because OIS can occur in the context of other serious conditions such as giant cell arteritis (GCA), clinicians should have a low threshold for workup (e.g., erythrocyte sedimentation rate, c-reactive protein, temporal artery biopsy) especially if associated GCA symptoms are present [1,10].
Based on the ophthalmic exam, imaging modalities such as OCT, fluorescein angiography, and B-scan may be obtained as needed. Fluorescein angiography can be helpful in determining the degree of ocular perfusion which would be demonstrated as a delay in choroidal filling and prolonged arteriovenous time, or evaluate complications of OIS, including neovascularization [11].
Treatment
The most important intervention in OIS is treatment of the underlying cause: internal carotid artery stenosis. In addition to reducing the risk of ocular complications, early management of the internal carotid artery stenosis may reduce the risk of overall mortality. As stated above, overall, five-year mortality in OIS has been estimated to be as high as forty percent [9]. Carotid stenosis may be treated with stenting, endarterectomy, or external-to-internal carotid bypass. The Carotid Endarterectomy trial demonstrated that in patients with severe, symptomatic carotid stenosis (defined as 70-99% occlusion) carotid endarterectomy provides a 17% risk reduction against ipsilateral stroke and 10% risk reduction for major or fatal strokes as compared to medical management [12]. However, carotid endarterectomy is contraindicated in patients with occlusion >99% due to concern for perioperative complications such as embolism causing a stroke [10,13]. As in the case shown above, these patients often will have collateral vessels that bypass the severe carotid stenosis.
Panretinal photocoagulation (PRP) is effective for treating both anterior and posterior segment neovascularization. In addition, anti-VEGF therapy may be used to treat neovascular complications, including iris and angle neovascularization [14] and may be particularly useful when PRP is not feasible (e.g., in the context of poor fundus visualization due to VH). In addition, intravitreal steroids and anti-VEGF therapy can also be used to reduce any associated macular edema [10].
EPIDEMIOLOGY
|
SIGNS
|
SYMPTOMS
|
TREATMENT/MANAGEMENT
|
References
Noble PM, Dawoud SA, Han IC. Ocular Ischemic Syndrome. EyeRounds.org. July 26, 2022. Available from https://eyerounds.org/cases/337-ocular-ischemic-syndrome.htm

Ophthalmic Atlas Images by EyeRounds.org, The University of Iowa are licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License.