33-year-old male with painful vision loss in the setting of sepsis and endocarditis
INITIAL PRESENTATION
Chief Complaint: Painful unilateral vision loss
History of Present Illness:
A 33-year-old male presented to an outside hospital for suicidal ideation. He had felt unwell for several days, had decreased vision and pain in his left eye, and attributed his suicidality to pain. He had used methamphetamine several days prior. He was febrile and tachycardic, with leukocytosis and lactic acidosis.
He was admitted and started on empiric ampicillin/sulbactam. Two blood cultures resulted positive for Streptococcus pneumoniae, and his antibiotics were transitioned to ceftriaxone. A transthoracic echocardiogram revealed a mobile echodensity on the anterior leaflet of the mitral valve. A follow up transesophageal echocardiogram confirmed signs of vegetation on the mitral valve with mild mitral regurgitation. He was cleared by psychiatry for discharge once medically appropriate, though there was concern for mild delirium in the setting of systemic infection.
During his stay at the outside hospital, his ocular symptoms continued to worsen. A local ophthalmologist evaluated him one week after admission, and found hand motion visual acuity, panuveitis, and retinal infiltrates with hemorrhage in the left eye. He was transferred to the University of Iowa for further care.
Past Ocular History:
None
Medical History:
Medications:
Naproxen PRN
Allergies:
No known allergies
Family History:
No relevant family history
Social History:
Review of Systems:
Positive for active dental infection, otherwise negative except for what is detailed in HPI.
OCULAR EXAMINATION
Full extraocular motility in both eyes (OU), although some pain with movement of the left eye.
Normal OU
|
Right Eye |
Left Eye |
|
|
Lids/lashes |
Normal |
Reactive ptosis |
|
Conjunctiva/sclera |
Clear and quiet |
2+ diffuse injection |
|
Cornea |
Clear |
Clear |
|
Anterior Chamber |
Deep and quiet |
Deep, trace cell |
|
Iris |
Normal architecture |
Poorly reactive with 360-degree posterior synechiae |
|
Lens |
Clear |
Clear |
|
Vitreous |
Normal |
2+ vitritis |
|
Right Eye |
Left Eye |
|
|
Disc |
Normal |
Hazy view, grossly normal |
|
Cup-to-disc ratio |
0.4 |
Hazy view, grossly normal |
|
Macula |
200-micron whitish fluffy superficial lesion ~750 microns from the optic nerve along inferotemporal arcade |
Hazy view; large, deep, white infiltrate in the posterior pole with multifocal intraretinal hemorrhages |
|
Vessels |
Normal |
Tortuous, dilated |
|
Periphery |
Normal |
Hazy view; scattered intraretinal hemorrhages, subretinal whitening extending temporally and inferiorly, shallow subretinal fluid inferiorly |
Further testing for the patient included ocular coherence tomography (OCT), fundus photography, and ocular echography. OCT and echography were normal for the right eye (OD). OCT for the left eye (OS) was limited due to hazy media.
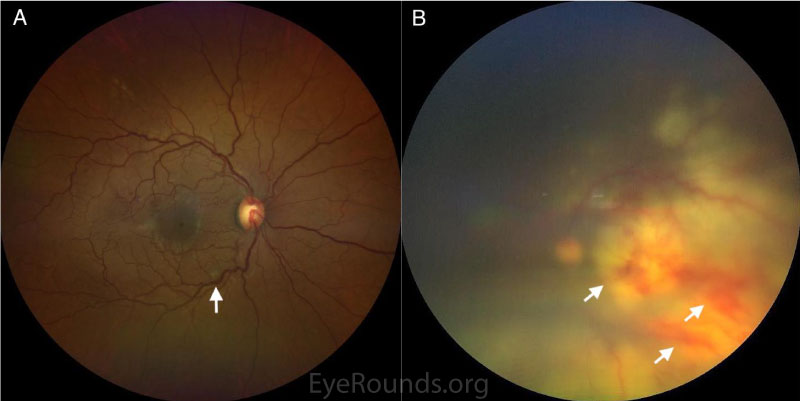
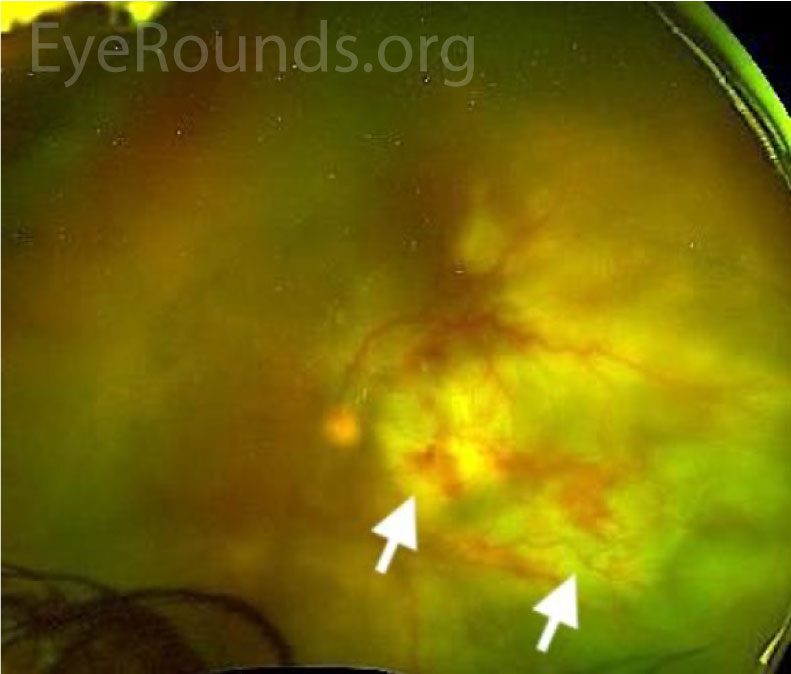
Differential diagnosis:
DIAGNOSIS: Endogenous endophthalmitis with subretinal abscess and rhegmatogenous retinal detachment secondary to Streptococcus pneumoniae septicemia
CLINICAL COURSE
Based on history, exam, and imaging, the patient was diagnosed with endogenous endophthalmitis and subretinal abscess of the left eye secondary to Streptococcus pneumoniae bacteremia.
He initially refused a vitreous aspirate, so he was treated with an intravitreal injection of vancomycin and started on atropine 1% daily and prednisolone acetate 1% every 2 hours. In case of possible acute retinal necrosis, he was also started on systemic valacyclovir. Broad-spectrum intravenous antibiotics were continued. Labs showed negative HIV, syphilis, Quantiferon, hepatitis B, and hepatitis C. A CBC showed mild leukocytosis, but blood cell morphology did not show evidence of leukemia or lymphoma.
After two days there was minimal improvement in the vitritis or subretinal lesion, and he agreed to undergo urgent diagnostic and therapeutic pars plana vitrectomy. Intraoperative inspection revealed a full-thickness macular hole and an inferotemporal posterior break consistent with rhegmatogenous retinal detachment. Endolaser was applied around the inferotemporal break and the inferior portion of the abscess. Intravitreal vancomycin was administered and silicone oil was placed. Stains and cultures of vitreous biopsy specimens were negative.
The infectious disease service recommended brain imaging to rule out any central nervous system infiltrate. Magnetic resonance imaging of the head and neck demonstrated a small right cerebellar infarct, likely due to embolic endocarditis. His neurologic exam remained normal throughout his stay.
Post-operatively, visual acuity improved to counting fingers. The subretinal abscess resolved, but there was residual choroidal whitening on fundus photographs (Figures 4, 5) and choroidal hyperreflectivity on OCT (Figure 6). The retina was attached, though the full-thickness macular hole persisted through the 2-week visit (Figure 6), after which he was lost-to-follow-up.
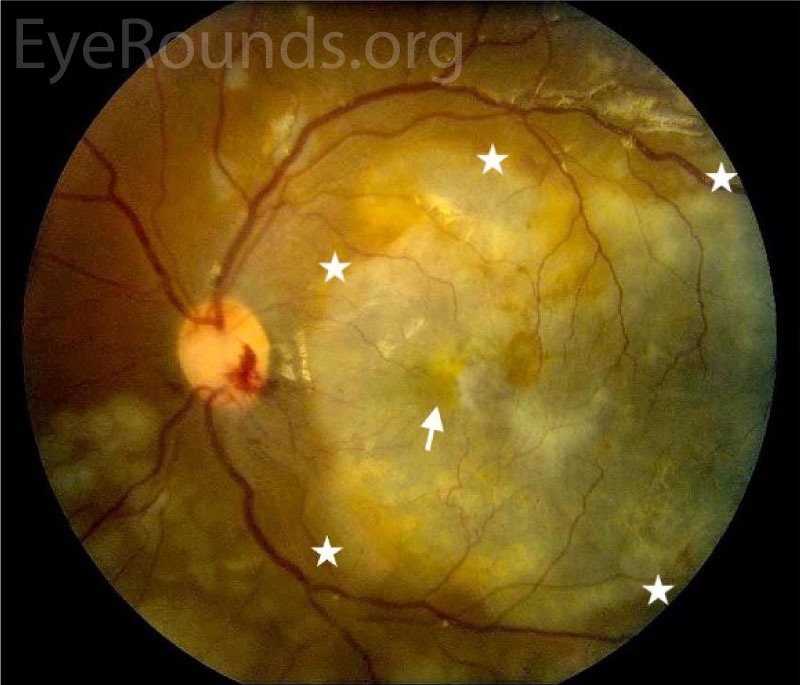
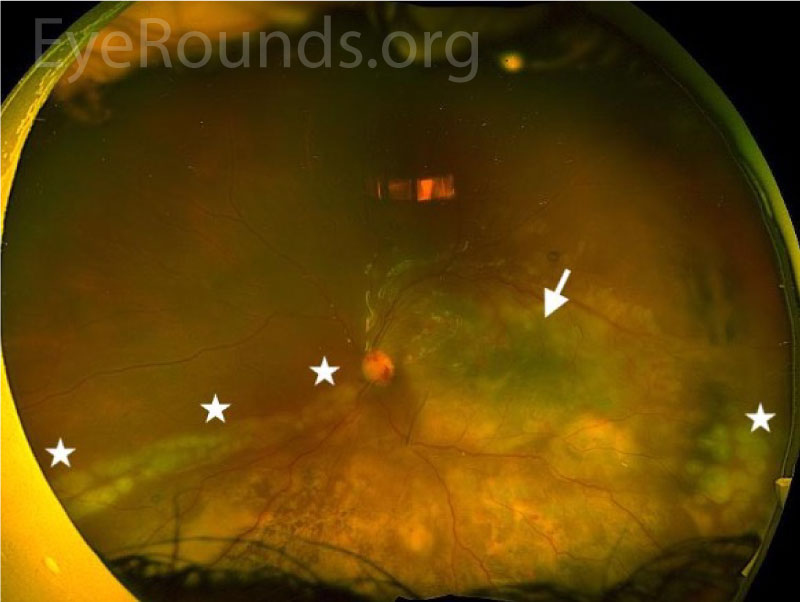
DISCUSSION
Etiology/Epidemiology
Endophthalmitis refers to severe inflammation involving the aqueous and vitreous fluids. Most cases of endophthalmitis result from exogenous introduction of a pathogen into the eye, often after trauma or ocular procedures. In contrast, endogenous endophthalmitis occurs due to seeding of the eye with a pathogen from the bloodstream [1].
Endogenous endophthalmitis poses a severe risk for vision loss and is often a complication of serious systemic disease [2]. Even in bacteremic patients, the development of endophthalmitis is rare: about 0.05% of cases of bacteremia in the United States are complicated by endophthalmitis [3,4]. Endogenous endophthalmitis may be more common in other areas of the world [5].
Any source of bloodstream infection can lead to ocular seeding. The most common sources of infection include infectious endocarditis, urinary tract infections, abdominal abscesses, meningitis, indwelling catheters, procedures that may cause transient bacteremia such as dental procedures or endoscopy, and intravenous drug use [2,6]. While all endogenous endophthalmitis occurs as a result of bacteremia or fungemia, sometimes the source is never identified. In many cases of endogenous endophthalmitis, the patient also has immunocompromise, such as recent hospitalization, early or advanced age, malignancy, diabetes, or use of immunosuppressive agents such as corticosteroids [7].
The types of organisms that cause endogenous endophthalmitis vary by region. In Western nations, Gram positive organisms from the Streptococcus and Staphylococcus families in addition to Candida yeasts are more common, whereas in Eastern nations there is a higher incidence of Gram negative organisms such as Klebsiella, Escherichia coli, Pseudomonas, and Neisseria [8].
Pathophysiology
Endogenous endophthalmitis occurs through the hematogenous spread of infectious agents to the posterior segment of the eye. Pathogens lodge in the choroid due to its high vascularity, then extend into the subretinal space. The infection can involve the retina and extend into the vitreous [9]. If the infection remains confined to the subretinal space, the associated vitreous cells are reactive rather than infectious in nature. Some infections, e.g. Candida, readily spread into the vitreous cavity where they form cellular aggregates.
Signs/Symptoms
Patients with endogenous endophthalmitis can be asymptomatic, symptomatic, or too systemically ill to report symptoms. Ocular symptoms can include eye pain, redness, floaters, and decreased vision. Signs on exam that may suggest endogenous endophthalmitis include anterior chamber inflammation (ranging from minimal to severe), posterior synechiae, cataract, vitreous cell/flare or aggregates, retinal vasculitis, and retinal hemorrhages. Whitish, elevated, subretinal lesions with somewhat indistinct borders in the proper clinical context are relatively specific for endogenous endophthalmitis.
Diagnosis/work-up
When there is no overt systemic infection, endogenous endophthalmitis can be difficult to diagnose, particularly if patients present with limited symptoms. Patients may be initially misdiagnosed with noninfectious uveitis or an alternative infectious uveitis such as Toxoplasmosis [10].
Endogenous endophthalmitis is a clinical diagnosis that can sometimes be confirmed with microbiological cultures. A rigorous search for systemic infection, including blood cultures, chest X-ray, urine cultures, and so forth is mandatory. Exogenous sources, such as occult trauma, recent surgery, or infection near the eye must be excluded [2]. Aqueous paracentesis may be diagnostically useful to exclude other entities such as CMV, HSV, or VZV. Serologies should be obtained to check for leukemia or lymphoma, HIV, syphilis, and TB. A vitreous aspirate or diagnostic PPV is usually appropriate, though neither may be necessary when the diagnosis is clear and the infection is mild or improving. However, a negative aspirate does not exclude endophthalmitis but rather serves to guide management.
Ocular imaging including OCT, fundus photography, and ocular ultrasonography can reveal subretinal abscesses, intraretinal and vitreous extension and inflammation, and associated retinochoroidal thickening or retinal detachment. Fluorescein angiography is occasionally useful to exclude mimicking conditions.
Treatment/Management/Guidelines
Endogenous endophthalmitis is considered a medical emergency, as it is often vision-threatening and results from infection that may be life-threatening.
Intravenous broad-spectrum antibiotics (and/or antifungals) and empiric intravitreal antimicrobials are usually appropriate first line therapy.
Systemic antimicrobial agents alone are sometimes sufficient to treat endogenous endophthalmitis, and they are generally necessary for treatment of systemic infection [11]. As with any septicemia, it is critical to start empiric broad-spectrum treatment early, then narrow the spectrum as cultures return. Cephalosporins are a common choice of antibiotic as they have some ability to cross the blood-retina barrier and are generally well tolerated. Other antibiotics with good ocular penetration include fluoroquinolones, some beta-lactams such as imipenem, and trimethoprim-sulfamethoxazole. Reasonable systemic options for fungal endogenous endophthalmitis include voriconazole, caspofungin, and amphotericin [12]. Finally, any sources of systemic infection should be addressed, such as removing unnecessary lines, drains, tubes, draining abscesses, and treating endocarditis.
Intravitreal antimicrobials are often used in conjunction with systemic antimicrobials [13]. The most commonly used intravitreal antibiotics include vancomycin with ceftazidime, and less commonly amikacin, meropenem, and moxifloxacin [11, 14]. In fungal infections, amphotericin, voriconazole, or fluconazole are most commonly employed [10]. Injections may need to be repeated, particularly in cases of fungal endophthalmitis if signs of infection persist [2].
In addition to antimicrobials, there is some controversial evidence that steroids may improve outcomes in patients with endophthalmitis. When used systemically or intravitreally, steroids may reduce inflammation and therefore mitigate visual damage [9].
There are no good studies to guide the use of pars plana vitrectomy (PPV) in endogenous endophthalmitis. The Endophthalmitis Vitrectomy Study (EVS) was performed decades ago and was limited to exogenous endophthalmitis after cataract surgery. Moreover, PPV techniques have advanced considerably since the EVS was conducted. Therefore, EVS conclusions do not apply to modern day cases of endogenous endophthalmitis. In general, there is not a consensus that endophthalmitis should be treated with early vitrectomy, although visual outcomes may be better along with a decreased need for enucleation or evisceration [15,16]. PPV is an effective method for obtaining larger-volume vitreous aspirates for culture. PPV can also be therapeutic by debulking the infectious load and reducing vitreous inflammation.
PPV is mandatory if there is a possible or definite rhegmatogenous retinal detachment (RRD). Retinal detachments in these eyes can be exudative, rhegmatogenous, and even tractional in nature; all three mechanisms can co-exist in the same eye. Ancillary lensectomy and/or scleral buckling may be indicated. If a retinal detachment is present, silicone oil should be placed because there is a high risk of proliferative vitreoretinopathy (PVR) and recurrent retinal detachment. It is generally best not to create a retinotomy to aspirate a subretinal abscess because of the high risk of PVR, but this is occasionally appropriate for diagnostic purposes in an eye with persistent infection that has not responded to prolonged systemic and intravitreal antibiotics.
Visual outcomes are highly variable and are generally worse in eyes with more virulent organisms, more chronic infections, abscesses involving the macula, and RRDs.
In conclusion, endogenous endophthalmitis is an ophthalmic and medical emergency that requires timely diagnosis and intervention to improve patient outcomes.
DEFINITION AND ETIOLOGY
|
DIAGNOSIS
|
SIGNS/SYMPTOMS
|
TREATMENT/MANAGEMENT
|
References
Sevcik K, Goldstein A, Russell J. Endogenous Endophthalmitis. EyeRounds.org. August 11, 2022. Available from https://eyerounds.org/cases/331-Endogenous-Endophthalmitis.htm

Ophthalmic Atlas Images by EyeRounds.org, The University of Iowa are licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License.