Chief Complaint: A 26-year-old male presents with acute horizontal binocular diplopia.
History of Present Illness: A 26-year old male with type 1 diabetes mellitus presented for evaluation of severe headache and double vision. His symptoms started 3 weeks prior to presentation with recurrent febrile episodes, nausea with emesis, a severe progressive headache, back pain, and neck pain. He initially sought care in local emergency departments several times and was treated for diabetic ketoacidosis (DKA) due to presumed viral illness. He reported that his blood sugars had been very difficult to control for the last several months despite being compliant with medications.
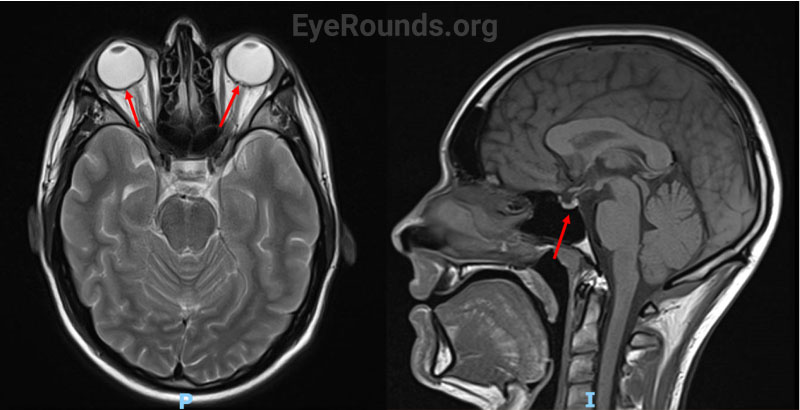
In the days prior to his presentation to ophthalmology, he developed binocular horizontal diplopia in addition to his other symptoms, which prompted further evaluation. He had no known exposure to mosquitos or tick bites, no transient visual obscurations or pulsatile tinnitus, and no history of sleep apnea. Magnetic resonance imaging (MRI) of the brain prior to presentation was obtained for the patient’s severe headache and lumbar back pain. This incidentally revealed a concave sella and posterior globe flattening of both eyes (Figure 1).
Past Ocular History: Refractive error, both eyes (OU)
Past Medical History:
Medications:
Allergies: None
Family History: Unremarkable
Social History:
Review of Systems: Unremarkable other than that noted in HPI
OCULAR EXAMINATION
| OD | OS | |
|---|---|---|
| Vitreous | Clear | Clear |
| Disc | Grade IV optic disc edema, peripapillary flame hemorrhages | Grade IV optic disc edema, peripapillary flame hemorrhages |
| Macula, vessels, and periphery | Nasal macula with retinal folds, tortuous veins | Nasal macula with retinal folds, tortuous veins |
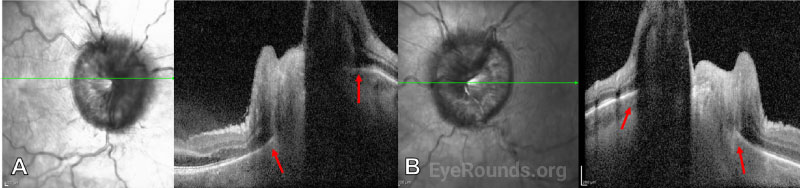
Together with the retinal folds, this suggests elevated intracranial pressure as the etiology of the optic disc edema.
Differential Diagnosis:
DIAGNOSIS: Cryptococcal Menigitis with Secondary Intracranial Hypertension
CLINICAL COURSE
Given the patient’s clinical exam findings of new onset cranial nerve six palsy and severe optic disc edema in both eyes, he was promptly referred to the Emergency Department. A lumbar puncture was performed, with CSF opening pressure measured at 36 cm H2O, which immediately improved the patient’s headache and back pain. The cerebrospinal fluid (CSF) was sent for comprehensive infectious analysis, which was positive for Cryptococcal antigen with a titer of 1:320. He had testing for syphilis and HIV, which were both negative. The patient was initially started on treatment with Acetazolamide. Infectious Disease was consulted for intitiation of treatment for Cryptococcal menigitis, and they recommended prompt antifungal treatment with Amphotericin B and oral Fluconazole. The patient was admitted and monitored during treatment. He developed an acute kidney injury with mild acidosis that was self-limited, but otherwise tolerated the treatment well.
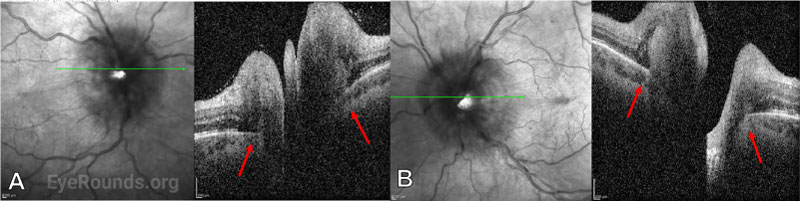
Subsequent examination two days after initial LP and initiation of antifungal treatment showed improvement of the optic disc edema to grade III in both eyes. On OCT, Bruch’s membrane configuration changed from angled into the eye to angled out, indicating a decrease in the pressure gradient between the retrobulbar space and the vitreous (Figure 5). The patient continued antifungal therapy for three weeks along with serial LPs, during which time his symptoms continued to improve. His extraocular motility returned to normal and within 1 month, and the optic disc edema was reduced to grade II in both eyes at that time. Over subsequent weeks of follow-up, he reported no recurrence of symptoms of increased ICP. Visual function and perimetry remained stable, and optic disc edema continued to improve. After completing induction therapy, his antifungal regimen was changed to fluconazole monotherapy for a treatment duration of one year.
DISCUSSION
Etiology/Epidemiology
Cryptococcal meningitis (CM) is the world’s most common cause of fungal meningitis and extrapulmonary cryptococcus.[1-3] There are over 220,000 annual cases, more than 181,000 annual deaths worldwide, and around 3,000 cases in the United States each year.[1] The two most prominent species that cause human pathogenesis are Cryptococcus neoformans and Cryptococcus gatti.[2] Cryptococci are encapsulated fungi that survive mainly in the environmental resevoirs of bird feces, soil, and decaying organic matter.[2] These capsules are then inhaled by humans, allowing the fungi to colonize the lungs and disseminate across the blood-brain barrier to cause meningitis.[2] Cryptococcal capsules have high virulence and genetic variability that allows them to evade the macrophage-led host immue response.2
CM is a high-risk infection which requires prompt diagnosis and intervention. Mortality rate varies drastically between high- and low-income countries, with high-income areas averaging 9% and low-to-middle income areas averaging 55%.[2] Sub-Saharan Africa, which accounts for 70% of the world’s cryptococcal meningitis cases annually, reports an average mortality rate of 70%.[2]
The incidence of CM is significantly associated with compromised immune system function due to various causes including human immunodeficiency virus (HIV), autoimmune disease, diabetes mellitus (DM), cancer, organ transplant, chronic kidney disease, alcohol and drug abuse, other disease leading to immune deficiency, or immunosuppressive drugs. HIV is associated with 95% of CM cases in low-to-middle income countries and 80% of cases in high-income countries.[3] However, the incidence of CM among non-HIV-infected persons is rising in developed countries with access to antiretroviral therapy.[2] Incidence of CM is lower in HIV-negative individuals, however mortality in these patients tends to be higher due to delayed diagnosis.[4]
In patients with underlying disease who contract cryptococcal infection, individuals with DM comprise 10-20% of cases.[5] Within this group there is a mild male predominance and a median age of 60.5 years.[5,6] Uncontrolled DM can precipitate an immunocompromised state, and is one of the most common predispositions for opportunistic infections such as cryptococcosis in HIV-negative individuals.[5]
Pathophysiology/Signs/Symptoms
Cryptococcal infection can remain temporarily asymptomatic in immunocompetent individuals, but often leads to more rapidly disseminated and symptomatic disease in immunocompromised patients. Initial signs and symptoms of CM vary in timing of onset and in severity and include fever, headache, neck stiffness, photophobia, change in or loss of vision, papilledema, cranial nerve palsies, focal neurological deficits, and altered mental status or depressed level of consciousness.[7,8] The most common clinical presentations in patients that have underlying DM with cryptococcal meningitis are headache, fever, altered mental status, nausea, and vomiting.[2]
Elevated ICP in cryptococcal meningitis can arise from interstitial edema, hydrocephalus, or from impaired CSF drainage.9 Higher CSF fungal concentration is associated with higher ICP, which in turn is associated with mental status changes and mortality.[7] CM can affect the optic nerve either through inceased intracranial pressure (ICP) or, more rarely, direct invasion of the optic nerve.[7] Infiltration of the optic nerve or nerve sheath tends to present with a relatively rapid onset of vision loss compared to papilledema, which tends to present with a slower, more progressive onset. In addition, optic nerve infiltration will often show enhancement on an MRI scan performed with fat suppression.[7,8]
Depending on the underlying etiology of ICP and a patient’s immune status, vision loss has a variable time course and presentation in CM. Around 50% of CM patients experience some degree of vision loss, with papilledema underlying up to 30% of these cases.[10] Papilledema due to increased ICP can cause severe permanent vision loss. Symptoms and manifestations of pailledema may include blurry vision, sixth nerve palsy, transient visual obscurations, or pulse synchronous tinnitus. Patients rarely can present with severe papilledema and rapid vision loss. Less commonly, central vision can be affected by papilledema-induced changes including submacular fluid or hemorrhages, however these changes are often reversible.
Testing/Laboratory Work-Up/Imaging
CSF or blood culture is considered the gold standard for diagnosis and monitoring response to treatment. Routine culture does not differentiate between C. gatti and C. neoformans, although canavanine-glycine-bromothymol blue agar culture can be used to further distinguish species.[10] Microscopy using india ink staining provides a more rapid test result with result times ranging from a few hours to three days.[11]
Currently, the World Health Organization recommends cryptococcal antigen (CrAg) detection assays of serum or CSF using latex agglutination or lateral flow assay, as these methods provide high sensitivity (98.2%) and high specificity (96.8%).[11] CrAg assays are the preferred method for diagnosis of CM. If access to CrAg is not available, then the preferred diagnostic approach is lumbar puncture with CSF India ink.[11]
Elevated ICP occurs in more than 50% of cases with CM, and therefore, LP is recommended even in minimally symptomatic patients to assess ICP and need for serial LPs.[8,10] In addition to LP, a visual field test should be administered and often shows enlargement of the blind spot due to papilledema, with or without arcuate pattern loss. In the setting of CM or suspected CM, neuroimaging is used to distinguish papilledema due to raised intracranial pressure from other causes of optic nerve elevation such as intracranial mass.[8] Magnetic resonance imaging (MRI) of the head can show radiographic findings of increased ICP. OCT findings including anterior retinal projection, total retinal thickness, and RNFL thickness may correlate with ICP.[12] Ultrasonography can also be sometimes be used to identify sheath distension in patients with increased ICP.
Treatment/Management/Guidelines
In addition to visual, auditory, and other neurological damage, uncontrolled ICP contributes to the high rate of mortality in patients with CM.[13] Treatment for ICP and coincident papilledema due to CM often involves intermittent, temporary or permanent CSF diversion.[7] Lumbar puncture can provide immediate symptomatic relief and either reverse or prevent neurological outcomes such as headache, vision loss or blindness, and mental status changes. Early lumbar puncture (<24 hours) following admission or diagnosis is associated with decreased mortality.[14,15] Current guidelines for managemet of CM and elevated ICP include up to daily serial LPs until ICP normalizes, with follow-up LPs after antifungal therapy to monitor CSF sterility and ICP.[4] A temporary lumbar drain or extraventricular drain may be necessary in patients with extremely elevated ICP. The merits of surgical placement of a CSF shunt during an active infection is debated among physicians but may be appropriate for patients whose ICP fails to normalize with serial LPs.[10]
Current guidelines recommend a combination therapy of amphotericin B (0.7–1.0 mg/kg per day) intravenously with flucytosine 100mg/kg/day for 14 days.[15] Historically, flucytosine availability has been limited in many parts of the world, and its high cost creates a barrier to treatment in areas where it is available. In cases where flucytosine is unavailable, combination therapy with amphotericin B 0.7–1.0 mg/kg per day and fluconazole 800 mg/day is recommended.[11, 15] Voriconazole has also been studied as an alternative to flucanozole in combination with amphotericin B with similar results at both 800 mg/day and 1,200 mg/day.[15] If amphotericin B availability is limited or a 14 day course is not feasible, a 5-7 day course of amphotericin B with fluconazole 1,200 mg/day is recommended as an alternative. Lastly, if amphotericin B is unavailable, fluconazole monotherapy at 1,200 mg/day for 10-12 days is recommended.[15]
EPIDEMIOLOGY
|
SIGNS
|
SYMPTOMS
|
TREATMENT/MANAGEMENT
|
Hartness E, Mansoor M, Linton EF, Kardon RH. Bilateral optic disc edema associated with cryptococcal meningitis in a young diabetic male. EyeRounds.org. Posted May 18, 2023; Available from https://EyeRounds.org/cases/341-bilateral-disc-edema-cryptococcal-meningitis.htm

Ophthalmic Atlas Images by EyeRounds.org, The University of Iowa are licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License.