
The University of Iowa
Department of Ophthalmology and Visual Sciences
Diabetic retinopathy (DR) is a vascular disease of the retina which affects patients with diabetes mellitus. It is the number one cause of blindness in people between the ages of 20-64 in the United States. It is, therefore, a worthwhile topic for all medical students to review. Diabetes mellitus is extremely common, so it is not surprising that DR affects 3.4 percent of the population (4.1 million individuals). Of the millions of people with DR, nearly one-fourth have vision-threatening disease (AAO 2008).
The likelihood of developing diabetic retinopathy is related to the duration of the disease. Type 2 diabetes has an insidious onset and can go unnoticed for years. As a result, patients may already have DR at the time of diagnosis. Type 1 diabetics, on the other hand, are diagnosed early in the course of their disease, and they typically do not develop retinopathy until years after the diagnosis is made. The risk of developing retinopathy increases after puberty. Twenty years after the diagnosis of diabetes, 80% of type 2 diabetics and nearly all type 1 diabetics show some signs of retinopathy (Klein 1984a, Klein 1984b).
While these numbers are eye-opening, diabetics can decrease their risk of retinopathy and slow the progression of the disease after it has begun with tight glucose control (DCCTRG 1993). Glucose control also has the added benefit of decreasing risk for other end-organ complications of diabetes, so it is important that diabetic patients are educated on the topic. Time since diagnosis and extent of hyperglycemia are the most significant risk factors for the DR, but other risk factors for development and progression include hypertension, dyslipidemia, smoking, nephropathy, and pregnancy (AAO 2008).
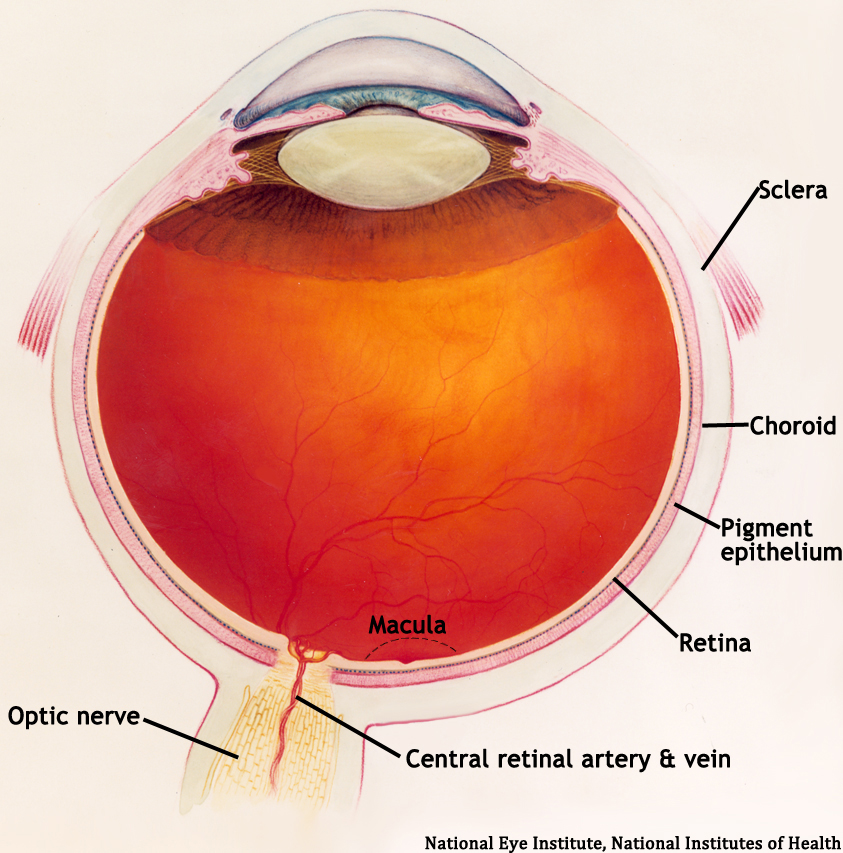
The retina is a multi-layered sheet composed of neurons, photoreceptors, and support cells. It is one of the most metabolically active organs in the body, and as a result, it is extremely sensitive to ischemia and nutrient imbalances (Frank 2004). A perfused retina is a happy retina.
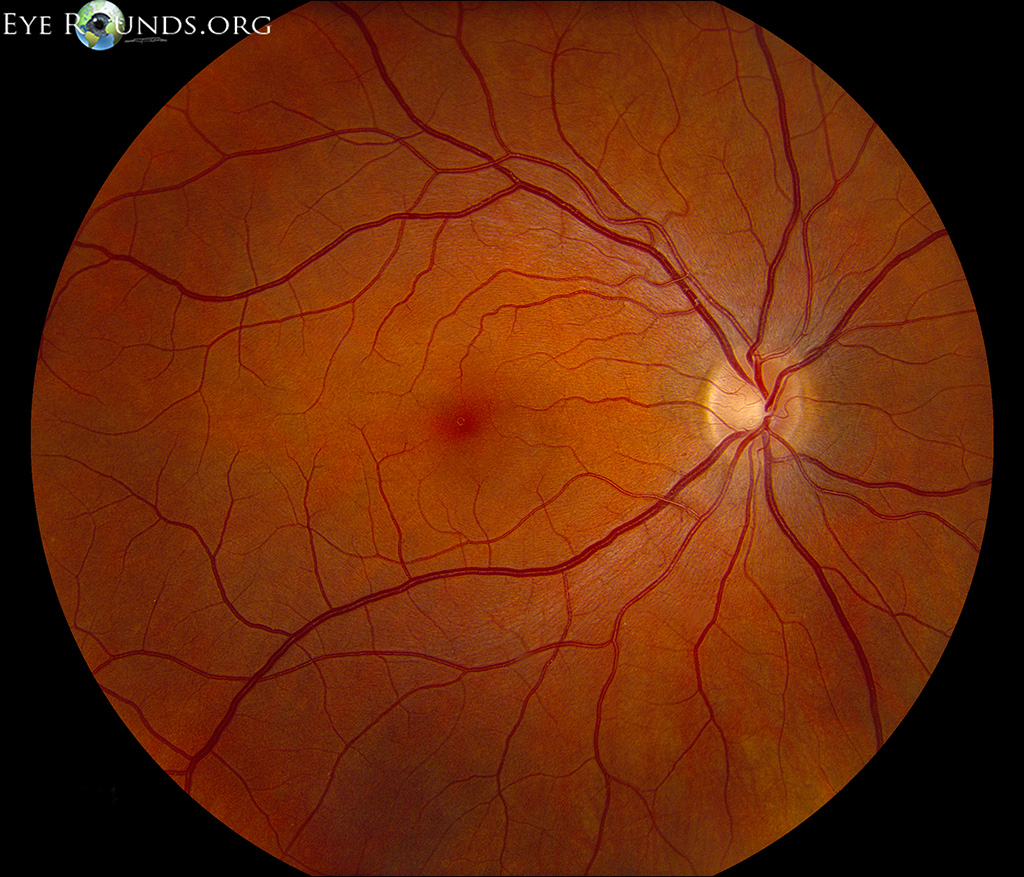
The outer one-third of the retina receives its blood supply from the choriocapillaris, a vascular network that lies between the retina and sclera. The inner two-thirds of the retina is supplied by branches of the central retinal artery, which comes from the ophthalmic artery (the first branch off of the internal carotid artery).
The central retinal artery exits out of the optic nerve, and its branches arch temporally both above and below the macula (the sensitive region of the retina responsible for central vision).
Although the exact pathophysiology of diabetic microvascular disease is unknown, hyperglycemia is thought to cause endothelial damage, selective loss of pericytes, and basement membrane thickening, all of which contribute to leaky, incompetent blood vessels
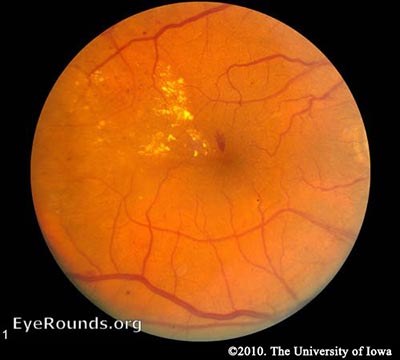
Diabetic retinopathy falls into two main classes: nonproliferative and proliferative. The word "proliferative" refers to whether or not there is neovascularization (abnormal blood vessel growth) in the retinaEarly disease without neovascularization is called nonproliferative diabetic retinopathy (NPDR). As the disease progresses, it may evolve into proliferative diabetic retinopathy (PDR), which is defined by the presence of neovascularization and has a greater potential for serious visual consequences.
Hyperglycemia results in damage to retinal capillaries. This weakens the capillary walls and results in small outpouchings of the vessel lumens, known as microaneurysms. Microaneurysms eventually rupture to form hemorrhages deep within the retina, confined by the internal limiting membrane (ILM). Because of their dot-like appearance, they are called "dot-and-blot" hemorrhages. The weakened vessels also become leaky, causing fluid to seep into the retina. Fluid deposition under the macula, or macular edema, interferes with the macula's normal function and is a common cause of vision loss in those with DR. Resolution of fluid lakes can leave behind sediment, similar to a receding river after a flood. This sediment is composed of lipid byproducts and appears as waxy, yellow deposits called hard exudates. As NPDR progresses, the affected vessels eventually become obstructed. This obstruction may cause infarction of the nerve fiber layer, resulting in fluffy, white patches called cotton wool spots (CWS).
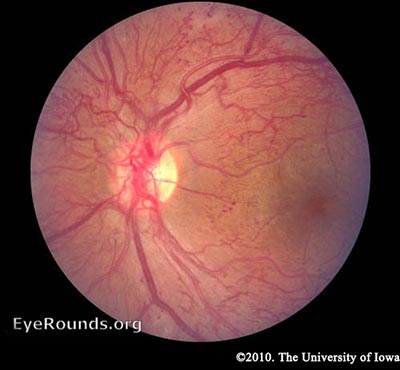
As mentioned earlier, the retina has a high metabolic requirement, so with continued ischemia, retinal cells respond by releasing angiogenic signals such as vascular endothelial growth factor (VEGF). Angiogenic factors, like VEGF, stimulate growth of new retinal blood vessels to bypass the damaged vessels. This is referred to as neovascularization. In PDR, the fibrovascular proliferation extends beyond the ILM. This may sound like a good idea, but the new vessels are leaky, fragile, and often misdirected. They may even grow off the retina and into the vitreous. As the vitreous shrinks with age, it pulls on these fragile vessels and can cause them to tear, resulting in a vitreous hemorrhage and sudden vision loss. These vessels may also scar down, forming strong anchors between the retina and vitreous causing traction on the retina. If enough force is created, a tractional retinal detachment may occur. This is another mechanism by which DR can cause sudden vision loss. If the retina is not re-attached soon, especially if the macula is involved, vision may be permanently compromised.
While the effects of neovascularization in PDR can be devastating, the most common cause of vision loss in diabetics is macular edema. Macular edema can occur in NPDR, but it is more common in more severe cases of DR due to the leakiness of the new blood vessels (Wani 2003).
Diabetics can also have problems located more anteriorly in the eye. The angiogenic molecules that are produced by the retina may float anteriorly, causing neovascularization of the iris. These vessels can grow into the angle of the anterior chamber where the trabecular meshwork, the drain of the eye, resides. This can obstruct outflow of aqueous fluid, raising intraocular pressure and causing acute glaucoma.
Patients usually do not experience symptoms until late in the course of the disease when treatment may be ineffective. Late symptoms of DR vary depending on the cause. Bleeding into the vitreous can cause sudden loss of vision. Macular edema and ischemia are two other mechanisms of decreased vision.
Screening for DR is incredibly important since most patients do not experience any symptoms until advanced stages of disease. If recognized early, the vision-threatening side-effects of DR can often be prevented with appropriate management. Recommendations for screening are different for type 1 and type 2 diabetics. Because many patients with type 2 diabetes have retinopathy at the time their diabetes is diagnosed, it is recommended that their annual dilated eye exams begin shortly after the diagnosis of diabetes is made. Type 1 diabetics should begin annual eye exams 3-5 years after their diabetes diagnosis (AAO 2008).
DR can progress rapidly during pregnancy. Pregnant women with diabetes should have a dilated eye exam prior to conception, early in the first trimester, and then every 3 months until delivery. Unlike pre-existing diabetes, gestational diabetes does not place patients at increased risk of DR and these patients do not require frequent eye examinations (AAO 2008).
A proper diabetic eye exam should always begin by gathering a thorough history from the patient. Ask about any visual symptoms and systemic issues which may impact their risk for DR such as pregnancy, blood pressure, cholesterol levels, and renal status. Additionally, make sure to check their last hemoglobin A1c to gain an idea how their blood glucose control has been over the past 3 months.
Examination should begin with visual acuity, intraocular pressure measurements, and a slit-lamp exam, including careful inspection of the iris for neovascularization. If neovascularization of the iris is suspected or the patient has elevated intraocular pressures, gonioscopy should be performed to assess the iridocorneal angle. Gonioscopy utilizes a special mirrored lens which allows you to view the angle and trabecular meshwork. This cannot be accomplished using the slit lamp alone. Next, the patient should have his or her pupils dilated for a thorough fundus exam.
NPDR is typically managed by optimizing the patient's general health. The best treatment for DR is prevention of its development and progression with tight glucose control (DCCTRG 1993). Patients should maintain a HbA1c ≤7%. Blood pressure management has also been shown to decrease disease progression (UKPDSG 1998) and patients should be counseled to stop smoking. Ophthalmologists should intervene if the patient has clinically significant macular edema (CSME) with NPDR. The causative microaneurysms are localized, often using fluorescein angiography, and then directly treated with laser therapy. If the leakage is more diffuse, a grid of light laser burns can slow the edema. Finally, several off-label medical options are available, such as intravitreous injections of triamcinolone and antibodies against VEGF such as Lucentis or Avastin.
Once a patient has developed PDR, there are several treatment modalities available. Treating macular edema in PDR is similar to treating NPDR. PDR, however, also has additional therapy options aimed at taming the growth of new, problematic vessels. The mainstay of treatment is panretinal photocoagulation (PRP), in which portions of retina are destroyed using thousands of laser burns while sparing the macula. It is hypothesized that this may reduce the amount of ischemic retina, and thus, reduce the production of angiogenic molecules. The treatment may sound extreme, but actually causes surprisingly little vision loss (Frank 1975). It has been found to be extremely effective, reducing the risk of severe vision loss by 50% (ETDRS 1987, Mohamed 2007) and resulting in regression of neovascularization in 30-55% of patients (DRS 1981, "Photocoagulation treatment" 1978).
Patients with non-resolving vitreous hemorrhages or severe traction causing retinal detachment may benefit from a vitrectomy. In this procedure, the vitreous gel and hemorrhage are removed from the eye and replaced with a saline solution.
PRP is advised for patients with vitreous hemorrhage and areas of neovascularization or in patients with large amounts of neovascularization of the optic nerve. It can also be considered in patients with severe NPDR to prevent progression to PDR.
Macular edema should be treated once it becomes clinically significant. CSME is diagnosed if the patient has at least one of the following criteria (Friedman 2009):
The optic disc measures 1500 µm in diameter on average and can be used to estimate distances along the retina.
Vitrectomy should be performed when a vitreous hemorrhage fails to resolve after 6 months or after 1 month in type 1 diabetics (Meredith 1998). The procedure is also indicated for certain tractional retinal detachments.
Fluorescein angiography (FA) may be used to definitively document retinal vessel occlusion and/or leakage. During an FA, a fluorescent dye is injected intravenously and a special camera takes fundus photos over several minutes while the vessels are being perfused. The test is best used to guide treatment of CSME, and not for diagnosis (AAO 2008).
Neovascularization in PDR can be seen on the slit lamp and dilated fundus exams and does not require any special testing.
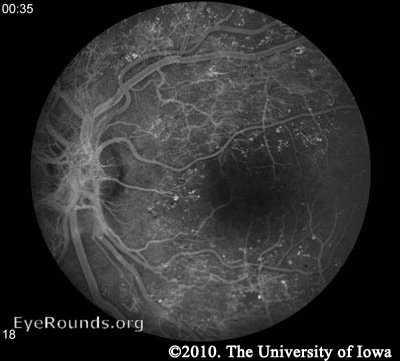
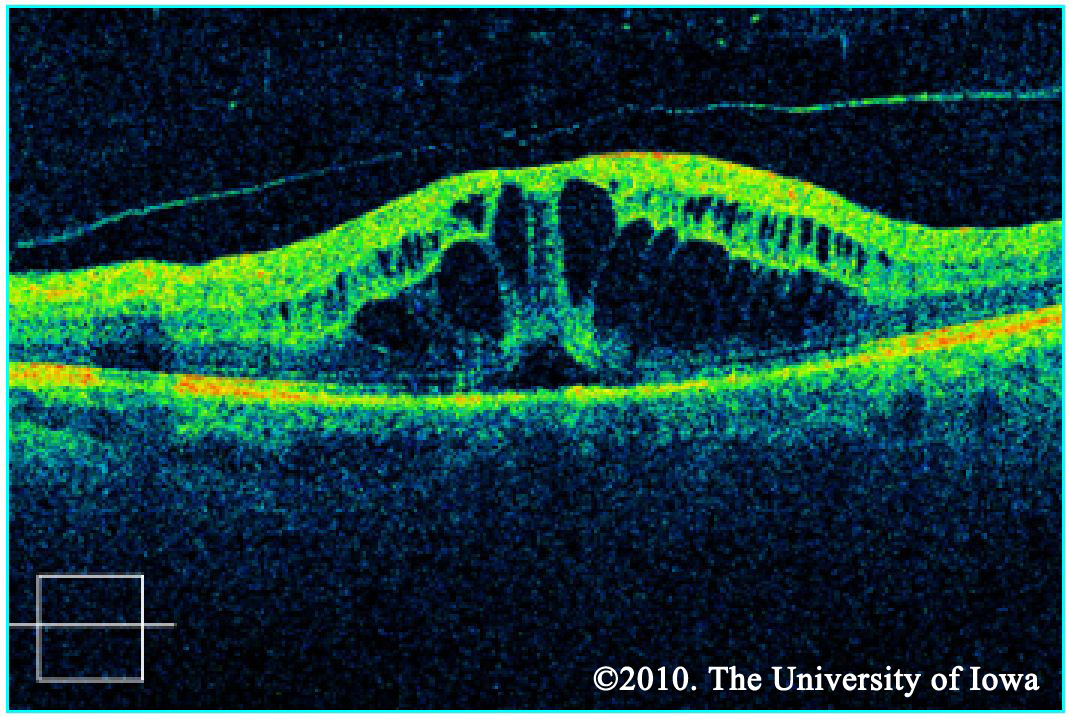
Macular edema may be noted on fundus exam, but is much more easily appreciated using optical coherence tomography (OCT). OCT is a laser imaging technique which produces an image showing the individual layers of the retina and the shape of the retinal surface. Fluid accumulation between layers can be appreciated as black patches on the scan and irregular retinal surfaces may reflect the etiology behind a patient's visual abnormalities. It should be noted, however, that in the ETDRS (the study which is used to support the treatment for CSME), OCT was not used. CSME was diagnosed only by clinical appearance.
Ultrasound is useful to detect retinal detachment when the fundus cannot be clearly seen on exam due to a dense cataract, vitreous hemorrhage, or other reasons.