
The University of Iowa
Department of Ophthalmology and Visual Sciences
September 1, 2015
Disclaimer: The medications discussed in this article are limited to those available in the United States. There is variability in availability from country to country. The medication cost included in each table is derived from the average cost of each medication found in local pharmacies in the Iowa City/Coralville, Iowa area as reported by GoodRx.com. Prices may vary by geographic area.
There are numerous risk factors for glaucoma, but the only one we can effectively treat is intraocular pressure (IOP). All medications used in glaucoma act to either decrease production of aqueous humor or increase its outflow, thereby reducing IOP. The goal of this article is to introduce the different classes of medications, their mechanisms of action, efficacy, and potential side effects. Medical management of glaucoma is an art form. The decision to start, stop, or adjust a medication is often a gray area and may be approached differently by different eye care providers. A PDF version of the medication tables included in this article may be downloaded. This is intended to be a used as an easily portable reference.
Four prostaglandin analogues are currently available for clinical use: latanoprost, bimatoprost, travoprost, and tafluprost (Table 1). In general, these medications are well tolerated, popular, and highly effective for most patients. Although the exact mechanism of action of this class of medications is not fully known, an increase in uveoscleral outflow is generally accepted as the primary mechanism. Some studies suggest prostaglandin analogues also increase trabecular outflow facility by regulating matrix metalloproteinases and remodeling the extracellular matrix within the trabecular meshwork, however the data supporting this theory is not as consistent as that describing uveoscleral outflow [1].
Prostaglandin analogues are dosed once every evening with peak effect 10-14 hours after administration, and more frequent dosing may actually lead to a paradoxical increase in pressure [1]. Studies show an IOP lowering ability of 25-32% for latanoprost, travoprost, and tafluprost and 27-33% for bimatoprost [2]. Latanoprost and travoprost are prodrugs that are activated after being hydrolyzed by corneal esterases. Prostaglandins are indicated for all types of open angle glaucoma, including primary open angle glaucoma, pseudoexfoliation glaucoma, pigmentary glaucoma, and normal-tension glaucoma. These medications are not as effective in primary congenital glaucoma and angle closure glaucoma [3]. Prostaglandin analogues are relatively contraindicated in patients with cystoid macular edema and in patients with inflammatory glaucoma due to the theoretical risk of worsening inflammation.
Side effects of prostaglandin analogues are mainly ocular. Hyperemia is common, but is minimized by the evening dosing. Increased iris pigmentation, observed in 33% of patients after five years, occurs more frequently in persons with hazel (yellow-brown) irides. Other side effects include periocular hyperpigmentation, hypertrichosis, hyperemia, and periorbitopathy (Figure 1). These effects appear to be reversible with drug discontinuation.
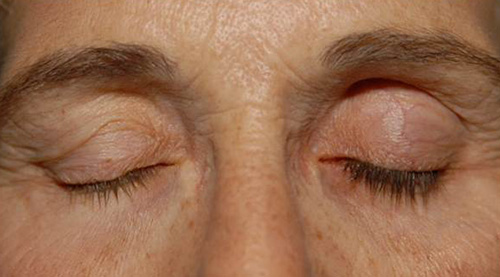
Table 1: Commonly used Prostaglandin analogues
| Generic | Brand |
Concentration |
Dosing |
Cost Generic |
Cost Brand |
Example Cap Color |
|---|---|---|---|---|---|---|
| Latanoprost | Xalatan® | 0.005% | QHS | $16/2.5ml | $140/2.5ml |  |
| Travaprost | Travatan® | 0.004% | N/A | $80/2.5ml | ||
| Bimatoprost | Lumigan® | 0.01%, 0.03% | N/A | $150/2.5ml (0.1%) | ||
| Tafluprost | Zioptan® | 0.0015% | N/A | $160/month |
β-blockers are popular, effective, generally well-tolerated, and indicated in all forms of glaucoma. Four β-blockers are currently available for clinical use: timolol, levobunolol, carteolol, and betaxolol, and can be divided into two subclasses of topical β-blockers: Non-selective and selective (Table 2). The non-selective β-blockers, timolol, levobunolol, and carteolol, target both β-1 and β-2 receptors, while the selective β-blocker, betaxolol, selectively targets only β-1 receptors [1]. The mechanism of action involves the blockade of sympathetic nerve endings in the ciliary epithelium, decreasing cyclic adenosine monophosphate (cAMP) production, and subsequently decreasing aqueous humor secretion by 20-30% during the day [3]. β-blockers have little IOP-lowering effect at night and are thus generally dosed once in the morning, or sometimes twice daily, especially when used in combination agents.
Patients taking a systemic β-blocker may have a diminished response to topical therapy. Prolonged use of β-blockers can result in tachyphylaxis. A reduced IOP response may also occur within weeks of starting treatment, as receptor saturation responds with up-regulation of the β-adrenergic receptor.
Treatment of one eye with β-blockers will sometimes lead to a decrease in the IOP of the contralateral, untreated eye, indicating there is a degree of systemic uptake with topical β-blockers. Recall that the β1-receptor has largely cardiac effects, and the β-2 receptor has largely pulmonary effects. Betaxolol, a β1 selective receptor antagonist, therefore, has fewer systemic respiratory side effects when compared to non-selective β-blockers, which target both β1 and β2 receptors. Though the efficacy of IOP-lowering is reduced in betaxolol for this same reason, betaxolol is considered to be safer for patients with respiratory or central nervous system (CNS) disease [3]. β-blockers have also been shown to decrease high-density lipoprotein (HDL) and increase cholesterol levels, though it is unclear the impact this may have on cardiovascular risk. Carteolol may have fewer effects on serum lipids [1]. Caution should be used when treating children with beta blockers, as they can reach high serum concentrations.
Systemic side effects may include bronchospasm, bradycardia, increased heart block, masking of hypoglycemic symptoms, decreased blood pressure, reduced exercise tolerance, depression, syncope, CNS depression, mood swings, and decreased libido. Abrupt withdrawal may worsen hyperthyroidism. Ocular side effects may include allergy, punctate keratitis, corneal anesthesia, and aggravation of myasthenia gravis.
Table 2: Commonly used Beta-Adrenergic Antagonists (β-blockers)
| Generic | Brand |
Concentration |
Dosing |
Cost generic |
Cost brand |
Example Cap Color |
|---|---|---|---|---|---|---|
Non-selective |
||||||
| Timolol | Timoptic® | 0.25%, 0.5% | QD-BID | $4/5ml (0.5%) | $160/5ml (0.5%) |  0.5% β-blockers |
| Levobunolol | Betagan® | 0.25%, 0.5% | $4/5ml (0.5%) | $58/5ml (0.5%) | ||
| Carteolol | Ocupress® | 1.0% | $12/5ml | N/A | ||
β1-selective |
||||||
| Betaxolol | Betoptic® | 0.25%, 0.5% | QD-BID | $50/5ml (0.5%) | $286/5ml (0.5%) |  0.25% β-blockers |
Two α2-adrenergic agonists are currently available for use: apraclonidine and brimonidine. These have replaced non-selective adrenergic agents which caused ocular vasoconstriction, pupillary dilation, and eyelid retraction via α1-adrenergic agonism. The α2-agonists decrease aqueous production and increase aqueous outflow, although their exact mechanism remains unclear. The α2-agonists are indicated for all forms of glaucoma, and some evidence suggests they may have neuroprotective effects, which could provide additional benefit in normal-tension glaucoma [4].
Apraclonidine is often used in the pre- and post-operative setting, particularly after laser or cataract surgery, as it is an effective short-term IOP lowering agent. Patients often develop topical sensitivity or tachyphylaxis, which limits its long term use. Further, the incidence of allergic reaction to apraclonidine is up to 40% and may include follicular conjunctivitis and contact blepharodermatitis. Fortunately, the cross-reactivity to brimonidine in patients with allergy to apraclonidine is minimal. Despite low incidence of true allergy to brimonidine, long-term intolerance is high (>20%) due to local adverse effects, i.e. hyperemia and blepharoconjunctivitis and even ectropion and granulomatous anterior uveitis. If a patient is on several drops and presents with the aforementioned signs or symptoms, regardless of the duration of therapy, it is reasonable to first suspect the α2-agonist as potentially contributing. Preservative free options exist and will be reviewed later in this article.
Both α2-agonists, apraclonidine and brimonidine, can lower IOP by 20-30% and are dosed BID or TID. Brimonidine is found in combination with timolol (Combigan©), dosed BID, or brinzolamine (Simbrinza©), dosed BID or TID. An absolute contraindication to brimonidine is use in children under 3-4 years of age as it may cross the blood brain barrier and result in fatal respiratory arrest, along with somnolence, hypotension, seizures, and derangement of CNS neurotransmitters. Apraclonidine is a safer alternative, as it does not cross the blood brain barrier. The α2-agonists are relatively contraindicated in patients taking monoamine oxidase inhibitors or tricyclic antidepressants.
Remember, α2-agonists are notorious for ocular side effects. Apraclonidine may cause irritation, pruritis, allergy, follicular conjunctivitis, dermatitis, eyelid retraction, ischemia, conjunctival blanching, ocular ache, photopsia, and miosis. Brimonidine may cause foreign-body sensation, eyelid edema, dryness, and ocular sensitivity/allergy, though less allergy compared to apraclonidine (Figure 2). Systemic side effects may include hypotension, syncope, vasovagal attack, dry mouth and nose, headache, anxiety, depression, and fatigue.
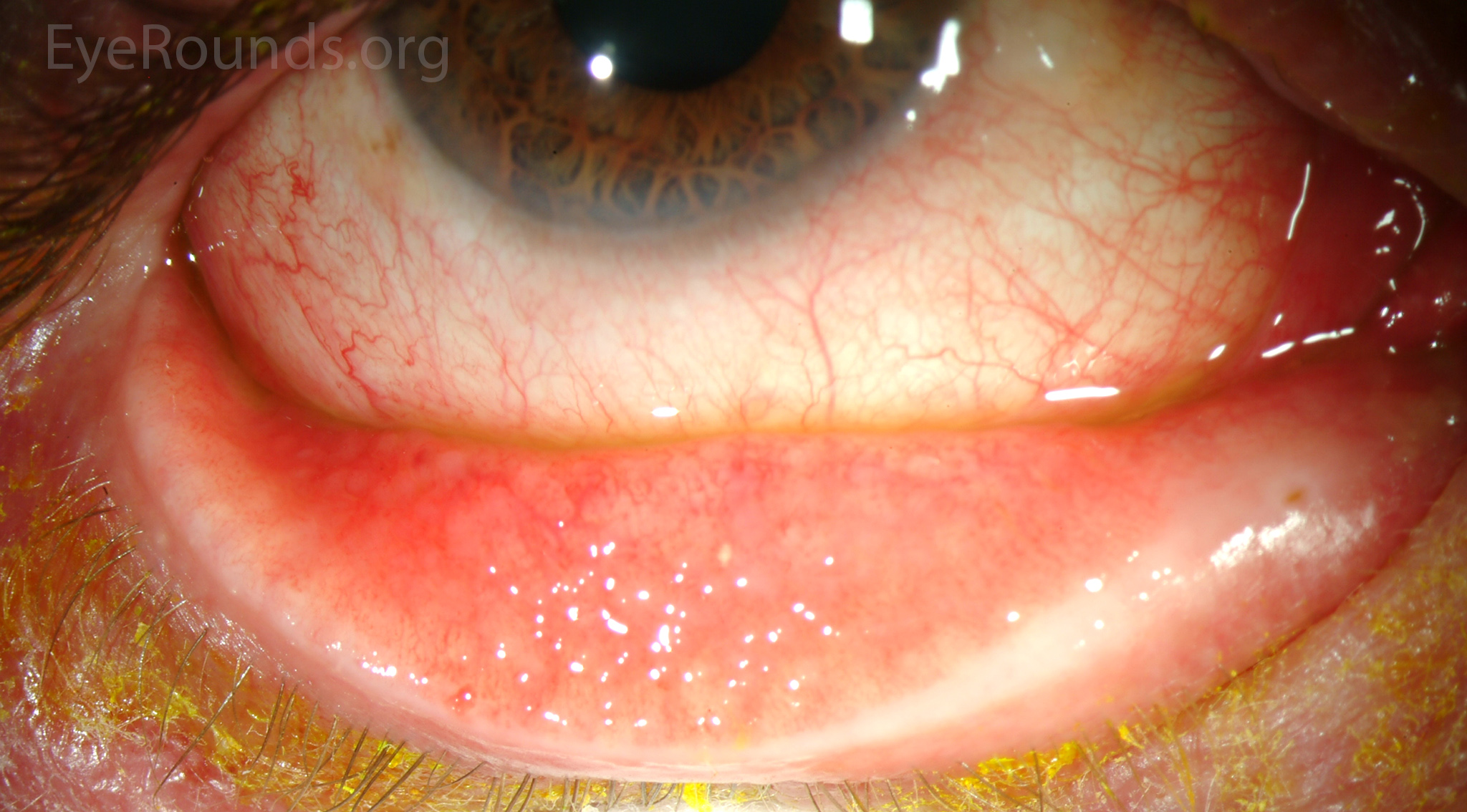
Table 3: Commonly used α2-Adrenergic Agonists
| Generic | Brand |
Concentration |
Dosing |
Cost generic |
Cost brand |
Example Cap Color |
| Selective | ||||||
| Apraclonidine | Iopidine® | 0.5%, 1.0% | BID-TID | $50/5ml (1%) | $152/5ml (1%) |  Apraclonidine |
| Brimonidine | Alphagan® | 0.1%, 0.2% | BID-TID | $9/5ml | $124/5ml (0.1%) |  Brimonidine |
Carbonic anhydrase inhibitors (CAIs) function as direct antagonists to ciliary epithelial carbonic anhydrase, an enzyme necessary for production of aqueous humor. More than 90% of this enzyme must be inhibited in order to decrease intraocular pressure [1]. Systemic CAIs, of which acetazolamide and methazolamide are the most common, have been available for decades, but their use in glaucoma has been limited due to the potential for serious side effects and the availability of alternative topical agents with fewer side effects. Topical CAIs (dorzolamide, brinzolamide) are generally better tolerated with fewer side effects and are commonly used as both an individual medication for glaucoma as well as part of several combination drops. Systemic agents have greater efficacy, with 30-50% IOP reduction compared to 15-20% IOP reduction with topical agents [5]. Of the systemic agents, acetazolamide is considered more effective than methazolamide, though it also has more side effects. Acetazolamide is eliminated in the kidneys, while methazolamide is metabolized in the liver.
Common adverse effects of topical CAIs include bitter taste and punctate keratopathy. Eyes with compromised endothelial dysfunction (e.g. Fuchs dystrophy) are at higher risk for corneal decompensation and should not be treated with topical CAIs. Some patients complain of burning with dorzolamide; this is less problematic with brinzolamide. Adverse effects of systemic CAIs are usually dose-dependent and include paresthesias of the fingers and toes, fatigue, loss of energy, and loss of appetite. Abdominal discomfort and bitter taste, particularly with carbonated beverages, are also commonly encountered. Rarely, patients may develop blood dyscrasias, including aplastic anemia, thrombocytopenia, and agranulocytosis. Hypokalemia may also develop, particularly in patients who are taking other diuretic medications (e.g. thiazides) (1).
Although CAIs are sulfonamide derivatives, they are generally tolerated in those with sulfa allergies [6]. In a large retrospective study by Lee et al. in 2004, high dose acetazolamide was used in patients with idiopathic intracranial hypertension as well as a self-reported sulfa allergy without serious adverse effects [7].
Table 4: Commonly Used Carbonic Anhydrase Inhibitors
| Generic | Brand |
Concentration |
Dosing |
Cost generic |
Cost brand |
Cap Color |
| Topical |  |
|||||
| Dorzolamide | Trusopt® | 2.0% | BID-TID | $25/10ml | $87/10ml | |
| Brinzolamide | Azopt® | 1.0% | N/A | $260/10ml | ||
| Systemic | Tabs are white or orange | |||||
| Acetazolamide | Diamox® | 250, 500mg | BID-QID | $56/120tabs (250mg) | N/A | |
| Methazolamide | Neptazane® | 25, 50, 100mg | BID-TID | $140/60 tabs (50mg) | N/A | |
Pilocarpine is the most commonly used cholinergic in medical practice. Pilocarpine decreases IOP by stimulating ciliary muscle contraction. This produces traction on the scleral spur by virtue of its attachment to the ciliary musculature. The displacement of the scleral spur leads to an increase in conventional (trabecular) aqueous outflow. The miosis induced by pilocarpine also improves outflow in eyes with angle closure glaucoma by pulling peripheral iris from the anterior chamber angle. Conversely, pilocarpine decreases uveoscleral outflow, which may cause a paradoxical rise in IOP. Pilocarpine is typically administered four times a day. The maximum IOP lowering effect occurs within two hours, with a reduction in IOP of approximately 20% [1].
The ocular side effects of ciliary muscle spasm and miosis result in poor tolerance of pilocarpine, particularly in young patients with high accommodative ability. Pilocarpine should be avoided in highly myopic patients due to the increased risk of retinal detachment. Other potential ocular side effects include cataract formation and corneal endothelial toxicity. Systemic toxicity is rare and includes activation of the parasympathetic nervous system including diaphoresis, lacrimation, salivation, gastrointestinal distress, and bronchospasm.
Echothiophate is an indirect-acting cholinergic medication rarely used today due to the side effect profile (redness, brown ache, pigmented iris cysts) and the existence of numerous, more effective, and better tolerated topical medications. Echothiophate is still available in pharmacies, and has the advantage of being more potent and dosed twice daily, though it is more expensive than pilocarpine. Unfortunately, pilocarpine is intermittently unavailable.
Table 5: Commonly used cholinergic agonists
| Generic | Brand |
Concentration |
Dosing |
Cost generic |
Cost brand |
Example Cap Color |
| Direct |  |
|||||
| Pilocarpine HCl | IsoptoCarpine | 1%, 2%, 4% | QID | $4/15ml (1%) | $94/15ml (1%) |
|
| Indirect | ||||||
| Echothiophate iodide | Phospholine iodide | 1.25% | QD-BID | N/A | $100/5ml | |
Combination drops are an effective way to decrease the drop burden for patients who require multiple topical medications. Available combinations in the US include Dorzolamide/Timolol, Brimonidine/Timolol, and Brinzolamide/Brimonidine. Additional well-tolerated combination drops are available outside the US but are not FDA approved. The biggest drawback for patients prescribed combination medications is the high cost.
Table 6: Commonly used combination medications
| Generic | Brand |
Concentration |
Dosing |
Cost generic |
Cost brand |
Example Cap Color |
| Dorzolamide/ Timolol |
Cosopt® | 2.0%/0.5% | BID |
$25/10ml | $150/10ml |  Dorzolamide/ timolol |
| Brimonidine/ Timolol |
Combigan® | 0.2%/0.5% | N/A | $135/5ml |  Brimonidine/ timolol |
|
| Brinzolamide/ Brimonidine |
Simbrinza® | 1.0%/0.2% | N/A | $135/8ml |  Brinzolamide/ brimonidine |
Numerous studies have documented the potential for ocular surface toxicity with long-term use of preserved glaucoma medications [8-10]. A prospective survey by Pisella et al. in 2002 found a higher incidence of ocular surface symptoms (e.g. redness, follicles, punctate keratopathy) in patients taking preserved eye drops versus non-preserved drops, and many of the minor adverse reactions reported by patients may be caused by the presence of preservatives [11]. Benzalkonium chloride (BAK) is a widely-used preservative found in many commonly used glaucoma medications and several BAK-free and preservative free alternatives exist across multiple classes of glaucoma medications. Unfortunately, the high cost of these medications is prohibitive for many patients. Insurance coverage is often a challenge, with most insurance companies requiring documented failure of preserved medications prior to use of any non-preserved option.
Table 7: Commonly Used BAK-Free Options
| Medication | Brand |
Concentration |
Preservative |
Cost brand |
| Alpha agonists | ||||
| Brimonidine | Alphagan-P® |
0.1%, 0.15%, 0.2% |
Purite© |
$30/5ml (0.2%) |
| Prostaglandins | ||||
| Travaprost Tafluprost |
Travatan-Z® |
0.004% |
sofZia© |
$160/2.5ml |
| Beta blockers | ||||
| Timolol maleate gel Timolol in Ocudose |
Timoptic-XE® |
0.25%, 0.5% |
Benzododecinium |
$200/5ml (0.5%) |
| Cholinergics | ||||
| Echothiophate | Phospholine Iodide |
1.25% |
Chlorbutanol |
$100/5ml |
| Combination | ||||
| Timolol/Dorzolamide | Cosopt PF® |
0.2%/0.5% |
None |
$110/mo |
Flanary WE, Myers (Provencher) LA, Alward WLM. Medical management of glaucoma: A primer. EyeRounds.org. posted September 1, 2015; Available from:
http://www.EyeRounds.org/tutorials/glaucoma-medical-treatment