Giant Cell Arteritis
(Temporal Arteritis)
Sohan Singh Hayreh, MD, MS, PhD, DSc, FRCS, FRCOphth
Professor Emeritus of Ophthalmology
Ocular Vascular Clinic
Department of Ophthalmology and Visual Sciences
The University of Iowa
Roy J. and Lucille A. Carver College of Medicine
Iowa City, Iowa
- This information is intended primarily for ophthalmologists. It is a summary of material published in peer reviewed ophthalmic journals. For more detailed information, please refer to the papers in the bibliography and the various cited articles in those papers.
- Dr. Hayreh does not give an opinion without personally examining a patient; he feels that to do so is unethical and also potentially dangerous.
INTRODUCTION
Giant cell arteritis (GCA) is an OPHTHALMIC EMERGENCY, because it carries a high risk of severe visual loss in one or both eyes - loss which is usually PREVENTABLE. Early diagnosis is the key to correct management and prevention of visual loss. GCA is also well-known for masquerading as other diseases.
We have conducted the following GCA related studies on patients seen in our Ocular Vascular Clinic, over the past three decades:
- In 363 patients, who had temporal artery biopsy done in our department for suspected GCA, we assessed the validity, reliability, sensitivity, and specificity of the signs and symptoms of and diagnostic tests for GCA.[1]
- In 170 patients with positive temporal artery biopsy for GCA, we studied the ophthalmic manifestations of GCA.[2]
- In 85 patients with visual symptoms due to GCA, we investigated the incidence of occult giant cell arteritis (i.e. GCA not associated with any systemic symptoms).[3]
- In 84 patients (114 eyes) with visual loss, we investigated the incidence and extent of visual improvement achieved by high-dose steroid therapy.[4]
- In 144 patients (271 eyes), we investigated the incidence and extent of visual deterioration while taking high doses of corticosteroid.[5]
- In 101 GCA patients and 218 patients with non-arteritic anterior ischemic optic neuropathy (AION), we investigated the usefulness of thrombocytosis and other hematologic tests in diagnosis of GCA and differentiation of arteritic AION from non-arteritic AION.[6]
- In 145 patients whose GCA was confirmed by temporal artery biopsy, we investigated various aspects of corticosteroid therapy in the management of GCA in a 27-year planned study.[7]
These studies revealed much valuable information on GCA, which is helpful in its early diagnosis and management. They also showed that the controversy on diagnostic criteria and management of GCA is caused by the very different perspectives of GCA of rheumatologists and ophthalmologists[7,8] - rheumatologists essentially deal with patients with rheumatologic symptoms, while ophthalmologists see GCA patients with the far more serious manifestation of visual loss, or patients who lose vision without having any rheumatologic or other systemic symptoms at all – i.e., occult GCA.[3] Following is a brief summary of the information provided by our studies and relevant information from a review of the literature.[7]
AGE AND SEX OF PATIENTS WITH GCA
The age ranged from 56 years to 93.4 years (median 75.8 years).[1] Thus, GCA is a disease of middle-aged and elderly persons and not of young persons. Epidemiological studies have shown that the higher the age, the greater the prevalence of GCA. One such study showed that in persons aged 60-69 years the prevalence is 33 per 100,000 persons; in those over 80 years old, 843 per 100,000.
Sex distribution in our study was 27.4% men and 72.6% women. Thus, GCA is seen far more frequently in women than in men.
There is evidence that GCA is far more common among Caucasians than other races.[7] These racial differences suggest a genetic predisposition for development of GCA.
DIAGNOSTIC PARAMETERS
Our study, in GCA patients with positive temporal artery biopsy, showed the following validity, reliability, sensitivity, and specificity of the signs and symptoms of and diagnostic tests for GCA [1]:
Systemic symptoms: In our patients with positive temporal artery biopsy, systemic symptoms and signs included headache in 56%, anorexia/weight loss in 52%, jaw claudication (that is, the jaw hurts whenever they eat) in 48%, malaise in 38%, myalgia in 29%, fever in 26%, abnormal temporal artery in 20%, scalp tenderness in 18%, neck pain in 16% and anemia in 13%.[1]
On the other hand, 21.2% of the patients with visual loss and positive temporal artery biopsy for GCA had no systemic symptoms of any kind whatsoever at any time and visual loss was the sole complaint, i.e. they had occult GCA.[3] Therefore, absence of systemic symptoms and signs does not rule out GCA - an extremely important point to be borne in mind, because in many patients the diagnosis of GCA is dismissed straight away if they have no systemic symptoms and signs of GCA.
Hematologic tests: Our studies[1,6] have shown that the following three hematologic tests are key for the diagnosis of GCA.
- Erythrocyte sedimentation rate (ESR): A high ESR is traditionally emphasized as a sine qua non for the diagnosis of GCA. It is also well-established that estimation of ESR is an important test in diagnosis of GCA. In our study, in patients with positive temporal artery biopsy for GCA, it varied between 4 and 140 mm/hr (Westergren) (median 87.5 mm). We also evaluated ESR in 749 normal persons in whom the ESR ranged from 1-59 mm/hr (median 11 mm). We found that ESR levels increase with age and are also higher in women than in men. In the literature highly variable numbers are given for the normal values: most laboratories described it in men <10 mm/hr and in women <20 mm/hr; Miller et al.[9] put forward a formula to calculate the normal ESR: in men age divided by 2 and in women age+10 divided by two. Our study suggested that a useful cutoff criterion for normal ESR is <30 mm/hr in men and <35 mm/hr in women, with a sensitivity and specificity of 92%.
- With the ESR values in our study varying between 4 and 140 mm/hr in GCA patients and 1 and 59 mm/hr in normal persons, there is an overlap in lower levels of ESR between the two groups. Thus, the most important fact to remember is that normal ESR does not rule out GCA. GCA is missed in a number of patients simply because of the universal misconception that every patient with GCA must have a high ESR.
- C-Reactive protein (CRP): Our studies indicate that estimation of CRP (an acute phase plasma protein of hepatic origin) is a highly reliable, reproducible and rapid test. CRP reaches abnormal levels within 4-6 hours and can increase up to 1,000 times, and also shows a much more rapid response to treatment than the ESR. Unlike ESR, it is not influenced by age, sex or hematologic factors. It generally runs parallel with the ESR; however, in some cases, CRP is not elevated when ESR is. This dichotomy between the two tests is very helpful when ESR is elevated due to conditions unrelated to GCA. Normal value is <0.5 mg/dl in our laboratory. In our study, in GCA patients it varied between 0.5 and 34.7 (median 4.35) mg/dl, and in the normal controls it was <0.5 to 3.3 (median <0.5) mg/dl.[1] The sensitivity and specificity of CRP in detecting GCA was 100% and 79-83% respectively. CRP is a very useful test.
- Thrombocytosis: In our study[6] of biopsy proven GCA patients, 60% had thrombocytosis (defined as a serum platelet count >400 X103/µl) and that was significantly more prevalent in GCA patients than in normal control population. Sensitivity and specificity of thrombocytosis for diagnosis of GCA was 60.4% and 97.5% respectively. There was no difference in the prevalence of thrombocytosis in GCA patients with and without visual loss, as well as among those with and without systemic symptoms of GCA. There was a significantly (p<0.0001) higher prevalence of thrombocytosis in patients with GCA and arteritic AION than in those with non-arteritic AION.
- Other hematologic tests: In addition to these, other hematologic tests can also be helpful. Anemia is a well-known finding in GCA patients. Our recent study[6] suggests that evaluation of white blood cell count and hemoglobin and hematocrit levels provides additional useful information, because GCA patients have significantly higher white blood cell counts and lower hemoglobin and hematocrit levels than those without GCA.
In conclusion, the combined information provided by ESR, CRP, platelet and white blood cell count and hemoglobin and hematocrit levels is highly useful in diagnosis of GCA, although none of them individually is 100% sensitive and specific.
Temporal artery biopsy: This is considered the definite criterion for diagnosis of GCA, and in our studies we used that as our "gold standard" for GCA diagnosis. In 76 of 363 patients we did temporal artery biopsy on the second side when biopsy on one side was negative but there was a high index of suspicion for GCA from symptoms and signs; 7 of the 76 showed a positive biopsy on the second side. Thus, a total of 106 patients had a positive temporal artery biopsy.[1] None of our patients with negative temporal artery biopsy on follow-up developed evidence of GCA. Thus, so far we have never had a patient with a false-negative biopsy. We feel this is because we remove at least a one inch piece of temporal artery and do complete serial sectioning, to make sure that examination of "skip areas" does not lead to false-negative results which have been reported in the literature. For example, in one case we cut 300 sections and only one of them showed a typical GCA lesion.
I have discussed at length elsewhere the various issues regarding temporal artery biopsy.[7] The following two questions are frequently asked:
- Should temporal artery biopsy be done on one or both sides, and, if on both sides, should it be done simultaneously, or on second side only if the first is found to be negative?Because of the risk of complications following temporal artery biopsy and the infrequent need to do it on the second side to establish the diagnosis, I find no justification for doing biopsy on both sides at the same time.
- Does steroid therapy alter the temporal artery biopsy results? Most available evidence indicates that it does not.[7] I feel that in patients with a strong index of suspicion of GCA, it is dangerous to withhold steroid therapy till the biopsy results are available, because there is every possibility that the patients may suffer irreversible visual loss in one or both eyes before the biopsy results are available.
Clinical criteria most strongly suggestive of GCA: In our study, the odds of a positive temporal biopsy were 9 times greater with jaw claudication, 3.4 times with neck pain; 2.0 times with ESR 47-107 mm/hr relative to those with ESR <47 mm/hr and 3.2 times with CRP >2.45 mg/dl compared to CRP <2.45 mg/dl, and 2.0 times when the patients were aged >75 years as compared to those <75 years.[1] Other signs and symptoms did not differ significantly from those with negative biopsy.
Thus, we found the following set of criteria most helpful: Jaw claudication, CRP >2.45 mg/dl, neck pain and ESR > 47 mm/hr (Westergren). CRP is more sensitive than ESR and a combination of the two provides best specificity (97%) for diagnosis of GCA.
American College of Rheumatologists' Criteria For Diagnosis of GCA
For diagnosis of GCA, the following 5 criteria are advocated by the American College of Rheumatologists[10] as the "gold standard": (1) age > 50 years at onset, (2) new onset of localized headache, (3) temporal artery tenderness or decreased temporal artery pulse, (4) elevated ESR of > 50 mm/hour, and (5) positive temporal artery biopsy for GCA. They state that: "A patient shall be classified as having GCA if at least 3 of these 5 criteria are met." But in the American College of Rheumatologists[10] study, 18 of 214 (8.4%) patients' temporal artery biopsy was either negative for GCA (15) or not done (3), and that study advocated that in such cases new headache and scalp tenderness or nodules be "used as a surrogate".
There are some differences in the diagnostic criteria for GCA between our study[1,3,6] and those advocated by the American College of Rheumatologists[10]; I have discussed them in detail elsewhere.[7] The reason for the differences between the two studies is the difference in their patient populations, and that includes the following:
- All of our patients had temporal-artery-biopsy-confirmed GCA which is not true in the American College of Rheumatologists'[10] study.
- Since the American College of Rheumatologists'[10] study was conducted by rheumatologists, it would seem their primary criterion of inclusion was the presence of rheumatologic systemic symptoms, while our study had an unbiased group of patients with a wide variety of symptoms, since the patients were referred to us by physicians from all the medical specialties in our large medical center.
- The rheumatologic study would not include patients with occult GCA, since they are unlikely to consult a rheumatologist, as they have no systemic symptoms. In our study 21.2% of the patients with visual loss had occult GCA.[3] That must make an important difference. Since the most serious complication of GCA is visual loss, the criteria advocated by the American College of Rheumatologists'[10] study may be adequate for diagnosing GCA patients with rheumatologic symptoms, but they are not good enough to prevent all GCA patients from going blind.
Thus, in conclusion, it is evident that the American College of Rheumatologists[10] study criteria are likely to result in some false-negative or false-positive diagnoses of GCA, risking visual loss.
Doppler tests in diagnosis of GCA: Some have advocated the use of these tests in the diagnosis of GCA. I do not find any real role of these tests in diagnosis of GCA as well as evaluation of ocular circulation in eyes with visual loss due to GCA. I have discussed problems with these tests at length elsewhere.[7]
OCULAR MANIFESTATIONS OF GCA
As mentioned above, visual loss is the most feared and irreversible complication of GCA. Therefore, ophthalmologists are likely to be the first physicians consulted by GCA patients with visual loss, especially those with occult GCA[3] who have no associated systemic symptoms at all.
Of a total of 170 GCA patients in our study, 50% presented in our clinic with ocular symptoms.[2] Of the 85 patients with ocular symptoms, both eyes were involved in 45% of the patients.
- Ocular symptoms: These were amaurosis fugax in 26%, visual loss of varying severity in 92%, diplopia in 7% and eye pain in 7%. These occurred in various combinations. Amaurosis fugax was the only presenting visual symptom in 10%. That indicates that amaurosis fugax in persons aged >50 years is a red flag for GCA.
- Visual acuity: It was 20/40 or better in 21%, 20/50 - 20/100 in 17%, 20/200 - 20/400 in 8%, count fingers in 15%, hand motion in 10%, light perception in 13% and no light perception in 15% (see Table below).
- Ocular ischemic lesions: These were AION in 76.4%, central retinal artery occlusion in 13%, cilioretinal artery occlusion in 25%, posterior ischemic optic neuropathy in 6% and ocular ischemia in 1%. Cotton-wool spots were seen in one third of the eyes. Peripheral triangular chorioretinal ischemic lesions were seen in 10 eyes. The various ocular ischemic lesions were seen in a variety of combinations.
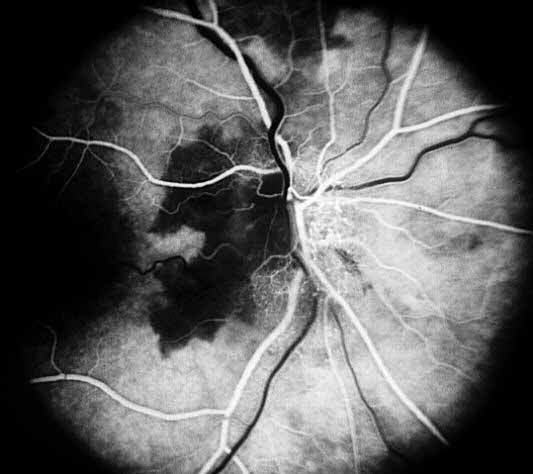
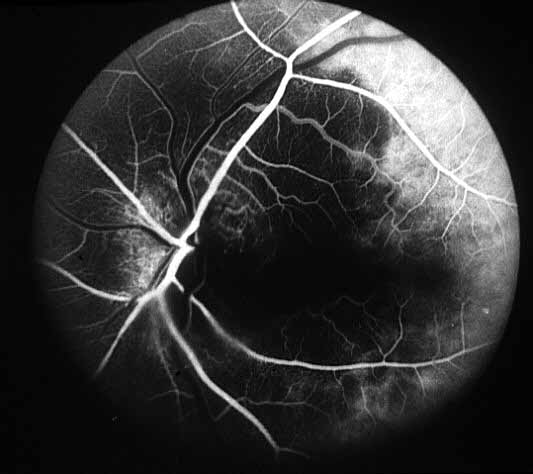
- Fluorescein fundus angiography: This is an extremely helpful test in diagnosis of GCA during the early stages of visual loss (see below) and also as a source of information about the cause of visual loss. In almost every patient with GCA in our series, it revealed occlusion of one or more of the posterior ciliary arteries.[2,11-14] (see Figure 1-b) When central retinal artery occlusion was present, there was almost always associated posterior ciliary artery occlusion as well; this is because the central retinal artery and posterior ciliary artery often arise by a common trunk from the ophthalmic artery,[2,13] and when that common trunk is involved by GCA, the eye presents with evidence of occlusion of both central retinal and posterior ciliary arteries.
OCCULT GCA
In this condition, the patient has ocular symptoms and signs but NO systemic symptoms or signs of GCA at all. Thus, ocular involvement is the sole reason for consultation. In our study, 21.2% of the GCA patients with visual loss had occult GCA. These patients run the greatest risk that the diagnosis of GCA will be missed and they will go blind in both eyes, because of the prevalent misconception among physicians that all GCA patients must have systemic symptoms and signs of GCA. The most common mode of presentation of occult GCA is as AION.
DIFFERENTIATION OF ARTERITIC FROM NON-ARTERITIC AION
AION is by far the most common cause of visual loss due to GCA and was seen in our study in 76.4% of eyes with visual symptoms associated with GCA.[2] For some unknown reason the most common ocular artery to be involved by GCA is the posterior ciliary artery.[2,7,11-14] In fact, in our studies, all patients with visual loss due to GCA showed evidence of posterior ciliary artery occlusion on fluorescein fundus angiography. That has also been demonstrated by histopathologic studies of eyes gone blind due to GCA.[7,13] Posterior ciliary arteries are the main source of blood supply to the optic nerve head.[3,14] Therefore, posterior ciliary artery occlusion in GCA results in ischemia of the optic nerve head and development of AION. Quite often the visual loss and/or progression in visual loss is discovered on waking up from sleep in the morning or from a nap, because the fall of blood pressure during sleep acts as the final insult to produce ischemia of the optic nerve head.
The most important fact in this context is that AION is a common disease in the middle-aged and elderly population and etiologically is of two types: (i) arteritic AION is due to GCA, and (ii) non-arteritic AION due to other causes. Non-arteritic AION is the more common of the two and is one of the most prevalent, visually crippling diseases in the middle-aged and elderly. Although the two types of AION have a similar clinical picture and presentation, their management is entirely different. Arteritic AION is an OCULAR EMERGENCY because of imminent danger of bilateral total blindness, which is usually PREVENTABLE; with early and adequate treatment these patients usually should not lose any further vision. In sharp contrast to that, so far there is no proven therapy available for non-arteritic AION.[14]
Therefore, once patients aged over 55 years are diagnosed as having AION, the first, crucial step is to identify immediately whether it is arteritic or non-arteritic. I have seen more than a thousand patients with AION since 1970. From that experience, I have developed a number of criteria that can help us to make this differentiation. I have discussed this in detail elsewhere.[7,11,14]The criteria are:
DIFFERENTIATING CRITERIA
- Systemic symptoms of GCA: Typically, in GCA, there are aches and pains all over the body, malaise, anorexia, flu-like symptoms, weight loss, fever of unknown origin, headaches, jaw claudication, neck pain, anemia and other vague systemic symptoms; the patient feels that there is something wrong but cannot really pinpoint what is wrong with him/her. I have described above the prevalence of various systemic symptoms and signs seen in our study.[1] But it is extremely important to remember that 21% of our patients with GCA related visual loss had no systemic symptoms and signs of GCA, i.e. occult GCA.[3]
- Visual symptoms: I have described above the visual symptoms seen in our study. [2] In this regard, the most important visual symptom is amaurosis fugax which was seen by us in 26% of the eyes or 31% of the patients, and almost invariably preceded the visual loss. Thus, if a patient with AION has had amaurosis fugax, that is strongly suggestive of arteritic AION. Similarly, if a patient with GCA develops amaurosis fugax, that is an ominous sign of impending arteritic AION and requires immediate treatment.
- ESR, CRP, platelet count and other hematologic tests: I have discussed above their role in the diagnosis of GCA (see above).
- Early, massive visual loss: Our studies have shown that arteritic AION patients usually present with much worse visual loss than those with non-arteritic AION. This is evident from the following table, based on our data of visual acuity in arteritic and non-arteritic AION.
Arteritic AION |
Non-arteritic AION |
|
|---|---|---|
20/40 or better |
21% |
41% |
20/50 - 20/100 |
17% |
22% |
20/200 - 20/400 |
8% |
10.5% |
Count fingers |
15% |
18% |
Hand motion |
10% |
5% |
Light perception |
13% |
1.5% |
No light perception |
15% |
2% |
- Chalky white optic disc swelling:[2,11-14] During the acute phase, this was seen in 69% of eyes with arteritic AION[2] (see figure 2) but is very rare in eyes with typical non-arteritic AION. Thus, chalky white swelling of the optic disc is extremely suggestive of arteritic AION.
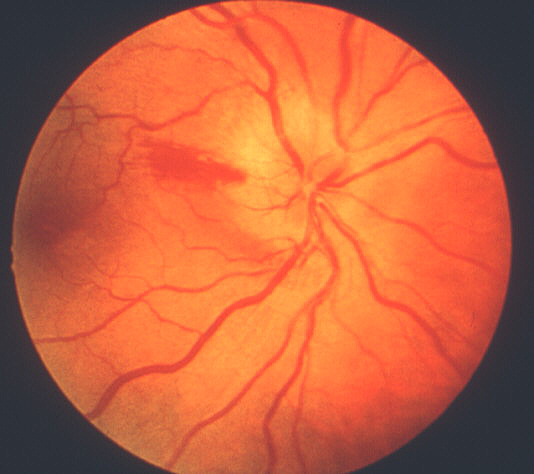
Figure 2: Fundus photograph of right eye during the early stages of AION, shows typical chalky white optic disc edema.
- Optic disc swelling associated with cilioretinal artery occlusion: If this combination is seen during the early stages of AION, that is very strongly suggestive of arteritic AION.[11-14] It is practically non-existent in non-arteritic AION.
- Posterior ciliary artery occlusion on fluorescein fundus angiography: If fluorescein angiography is performed during the very early stages, in arteritic AION there is always evidence of posterior ciliary artery occlusion with massive filling defect of the choroid[2,11-14] (see figure 1-b). This is extremely rare in non-arteritic AION. Therefore, angiography provides extremely useful information in diagnosis of GCA and arteritic AION and must always be performed in all AION cases to rule out arteritic AION.
- Temporal artery biopsy: Finally, temporal artery biopsy must be performed in every patient, even if one is absolutely confident about the diagnosis based on other clinical parameters. This is because patients with GCA require years of treatment with systemic corticosteroids, and are at great risk of developing serious systemic side-effects of steroid therapy. If a patient develops some serious complication(s) and blames the physician for that, the physician may have no scientific defense for the diagnosis, and the prolonged steroid therapy and consequent complications unless he/she has morphological confirmation of the diagnosis from a temporal artery biopsy. Various aspects of temporal artery biopsy are discussed above.[1]
Thus, I have found that, although almost none of these parameters is seen in 100% of the cases with GCA, the cumulative information supplied by all of them can almost always differentiate arteritic from non-arteritic AION.
For More information on anterior ischemic optic neuropathy please consult our web article on AION.
MANAGEMENT OF GCA PATIENTS
These recommendations are based on my experience of dealing with these patients for more than 30 years. In the treatment of GCA, one always has to keep in mind that, as stressed above, GCA is an ocular emergency. If there is a reasonable index of suspicion that the patient has GCA or arteritic AION, start treatment immediately. Do not wait for the results of temporal artery biopsy,because by the time the result comes, the patient may have developed irreversible visual loss in both eyes. If the biopsy is negative, treatment can be stopped without harm to the patient.
CORTICOSTEROID THERAPY
It is universally agreed that the treatment of choice for GCA is systemic corticosteroids. However, the exact regimen of steroids in GCA has become highly controversial. Perhaps the most important reason is that rheumatologists and ophthalmologists differ in their perspective on GCA. The former see patients with rheumatologic manifestations (many of them with polymyalgia rheumatica), and the latter see only GCA patients with visual loss and occult GCA. The steroid therapy regimen advocated by rheumatologists may be appropriate for polymyalgia rheumatica (with no risk of blindness), but I have found that there is no set formula of regimen of steroids for GCA patients because of the infinite variation between individuals.
The main issues of conflict and confusion are the dosage and duration of corticosteroid therapy required, and how to regulate steroid therapy? I have recently reviewed these and other aspects of steroid therapy in the management of GCA,7 and also reported findings of my 27-year planned clinical study on steroid therapy in GCA.[7] Following are the findings of this study:
Intravenous megadose steroid therapy (equivalent to 1 gram of Prednisone every 6-8 hours, repeated for 3-4 doses in the form of an intravenous drip) was initially given to 33% followed by oral steroids, while the rest had only the oral therapy. The median starting oral Prednisone dose was 80 mg/day, with 40% on 100-120 mg/day. I found that the most reliable and sensitive parameters to regulate and taper down steroid therapy were the levels of ESR and CRP, and NOT systemic symptoms. All patients were maintained at the high dose of Prednisone till both the ESR and CRP had stabilized at low levels (that usually took 2-3 weeks - CRP came down much faster than ESR - see figures 3 and 4 ); after that very gradual tapering of Prednisone was started, guided by the ESR and CRP levels only. The median time to reach the lowest maintenance dose of Prednisone at which the ESR and CRP stayed low and stable was 48.7 months, and the median lowest Prednisone maintenance dose achieved was 7 mg/day (interquartile range of 1 to 16 mg/day). There was no significant difference in the time to reach the lowest maintenance dose among patients with and without visual loss. My study showed that no generalization at all is possible for tapering down of Prednisone and there is no set formula because of the infinite variation between individuals. Only 10 of 145 patients were able to stop the therapy and maintain stable ESR and CRP levels during a median follow-up time of 2.43 years (inter-quartile range of 1 to 6 years). I found that the vast majority of GCA patients need a small dose of steroid therapy for years, if not for the rest of their life. The study showed no evidence that intravenous megadose steroid therapy was more effective than oral therapy in improving vision[4] or preventing visual deterioration5 due to GCA. In view of that new finding, I have now altered my intravenous regimen, giving only one initial intravenous loading dose, followed by oral Prednisone. My indications for intravenous corticosteroid therapy in GCA have always been any of the following ocular signs or symptoms:
- History of amaurosis fugax.
- Complete loss of vision in one eye.
- Early signs of involvement of the second eye, e.g., amaurosis fugax, visual field defects, optic disc edema, or sluggish retinal circulation in that eye. The objective is to try to prevent further visual loss in this high-risk group of patients by aggressive steroid therapy.
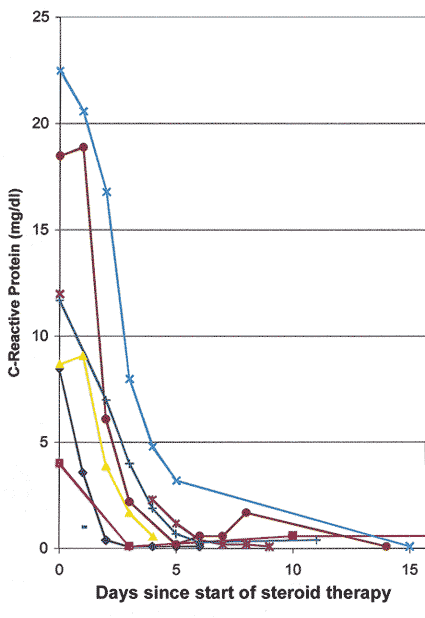
Figure 3: Graph of CRP levels in 6 patients with GCA, showing initial responses to high doses of systemic corticosteroid therapy.
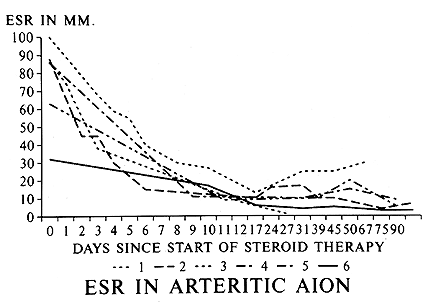
Figure 4: Graph of ESR (Westergren) rate in 6 patients with GCA, showing initial responses to high doses of systemic corticosteroid therapy. (Reproduced with permission from Hayreh[11])
Maintenance dose and duration of steroid therapy
My study has shown that determining the maintenance dose of steroid therapy is a slow, laborious, painstaking job, taking months or even years. The guiding principle, obviously, is to maintain the lowest level of ESR and CRP with the lowest dose of Prednisone. As mentioned above, my study showed marked inter-individual variation among GCA patients in: (a) the amount and duration of steroid required to control the active disease, (b) the time needed to reach a maintenance dose, (c) the maintenance dose required to keep the disease under control to prevent blindness, and (d) the total length of treatment.[4,5,7,8]Therefore, steroid therapy for GCA has to be individualized. I have found that most GCA patients require a virtually life-long, very small maintenance dose, which has little or no systemic side-effects.[7] A common mistake made by physicians in these cases is to taper the steroids down rapidly to a very low dosage and then discontinue it. There is a common belief among rheumatologists that GCA burns itself out in a year or two, and steroids can then be tapered off unless the patient develops systemic symptoms.[7,8] As discussed above, I have found this notion to be completely wrong, and can prove disastrous, because repeat temporal artery biopsy has shown evidence of active disease even after 9 years of steroid therapy.[7,8] The advice to base treatment on the clinical picture, rather than laboratory tests (i.e. ESR and CRP) may be true for polymyalgia rheumatica patients. But it can be dangerous for patients with GCA, who may lose vision irrevocably without developing any warning systemic symptoms at all; moreover, 21% of patients with visual loss have occult GCA. I have found that the only trustworthy and safe parameters to regulate the steroid therapy and to prevent visual loss are the levels of ESR and CRP, and nothing else.[4,5,7]
To have the full co-operation of the patient in the management of GCA, it is very important to explain the objectives of the treatment clearly to the patient. In my experience there is usually no useful recovery of visual function in the already involved eye.4 The patient needs to know this, to avoid having unrealistic expectations from the aggressive steroid therapy. The primary objective of treatment is to prevent loss of vision in the fellow eye. I have found that in spite of the starting intensive corticosteroid treatment, there are a few patients who are still likely to lose further vision during the first 5 days of treatment.5 However, I have not had anyone lose any more vision after that, if treated properly and adequately. Thus, the treatment is extremely effective, and about 5 days after the start of treatment one can almost guarantee that the patient will not lose any further vision. To maintain that vision, however, maintenance treatment with adequate oral corticosteroids is absolutely essential, and that is usually lifelong.
Alternate Day Corticosteroid Therapy in GCA
There is much misunderstanding among physicians about this mode of steroid therapy, and many of these patients are given alternate day therapy from the start. This mode of therapy has no place in the treatment of almost any active disease (including GCA) and is indicated only for maintaining suppression of disease activity and prevention of flare-up. Therefore, alternate day corticosteroid therapy has no place at all in the treatment of active GCA. In my experience, and that of others,[7] it is not even effective as a maintenance therapy regimen in GCA patients.
Corticosteroid Resistant GCA
Corticosteroid resistant GCA has been reported in the rheumatologic literature. I have reviewed the subject.[7] My study does not support this concept. I have had many patients referred to me over the years by outside physicians with that diagnosis; but when I treated them with adequate steroid doses, every single one immediately responded to steroid therapy. I feel that "corticosteroid resistant GCA" simply reflects a patient who has not been given an adequate amount of steroids. The basis for this erroneous impression may be mixing polymyalgia rheumatica and GCA patients in rheumatologic studies, with more of the former than the letter. An initial dose of Prednisone as low as 20 mg/day or even less may be adequate to manage polymyalgia rheumatica, but is totally inadequate to control active GCA and prevent visual loss.
STEROID SPARING AGENTS
I have reviewed the literature on this subject.[7] The most common steroid sparing agent discussed is Methotrexate. The unanimous conclusion of all the randomized clinical trials is that there is no real benefit in using methotrexate as a steroid sparing agent or to control GCA.
ASPIRIN OR ANTICOAGULANTS
Use of aspirin or anticoagulants in treatment of GCA to prevent ischemic lesions has been suggested by some, because of the presence of thrombocytosis in GCA (see above). I have reviewed the subject.[7] It seems the whole controversy on the association of thrombocytosis in GCA and ischemic lesions has emerged from a confusion between essential thrombocytosis (a chronic, progressive, myeloproliferative disorders of insidious onset with much higher platelet count) and reactive thrombocytosis (seen in GCA with a rather moderate increase in platelets). It is well known that patients with essential thrombocytosis have a high risk of thrombotic involvement of major vessels and the microcirculation. However, reactive thrombocytosis associated with GCA is not the same as essential thrombocytosis. There is no convincing evidence that ischemic manifestations occur as a direct consequence of reactive thrombocytosis in GCA. This would indicate that there is little justification for giving aspirin or other platelet anti-aggregating agents to prevent visual loss in GCA. Moreover, to date there are no studies which support the efficacy of anti-platelet agents or anticoagulants in the treatment of GCA to prevent blindness.
CONCLUSIONS
GCA is the most important medical emergency in ophthalmology, because of its high risk of visual loss, which is preventable if these patients are diagnosed early and treated immediately and aggressively. Thus, in patients aged over 55 years, if symptoms and/or signs suggest GCA or they have amaurosis fugax, AION, central retinal artery occlusion, cilioretinal artery occlusion or posterior ischemic optic neuropathy, always rule out GCA first before embarking on expensive and time-consuming investigations and treatments. If GCA is suspected, treat it as an emergency with systemic corticosteroids - temporal artery biopsy can wait. The management of GCA is highly complex, taxing and life-long; it should be undertaken with care and with the full understanding and co-operation of the patient and his/her internist.
VISUAL IMPROVEMENT OR DETERIORATION IN GCA WITH HIGH-DOSE CORTICOSTEROID THERAPY
Visual improvement: A review of the literature reveals many reports claiming visual improvement with systemic corticosteroid therapy. Some claim complete recovery or "dramatic improvement" of visual acuity in every eye, others in almost half and still others only rarely. In our study[4] of 114 eyes with visual loss due to GCA and treated with high-dose steroid therapy, only 4% had a variable amount of improved vision, none completely. The data also suggested that there is a better (p=0.065) chance of visual improvement with early diagnosis and immediate start of steroid therapy. We did not find a significant difference in the visual outcome between those treated with mega-dose intravenous steroid therapy versus high-dose oral steroid therapy.
Visual deterioration: Our study of 144 GCA patients (271 eyes) showed that although a few eyes (4% of the eyes or 6% of the patients) can develop visual deterioration while on high doses of steroid therapy, early, adequate steroid therapy is effective in preventing further visual loss in the vast majority.[5] When further visual deterioration occurred in spite of high doses of systemic corticosteroids, it almost invariably started within 5 days after initiation of high dose steroid therapy. Thus, early and aggressive steroid therapy can reduce the risk of further visual loss. There was no evidence that intravenous mega-dose steroid therapy was more effective than high-dose oral therapy in preventing visual deterioration.
References
- Hayreh SS, Podhajsky PA, Raman R, Zimmerman B: Giant cell arteritis: Validity and reliability of various diagnostic criteria. Am J Ophthalmol. 1997;123:285-96.
- Hayreh SS, Podhajsky PA, Zimmerman B: Ocular manifestations of giant cell arteritis. Am J Ophthalmol 1998;125:509-20.
- Hayreh SS, Podhajsky PA, Zimmerman B: Occult giant cell arteritis: Ocular manifestations. Am J Ophthalmol 1998;125:521-6,893.
- Hayreh SS, Zimmerman B, Kardon RH. Visual improvement with corticosteroid therapy in giant cell arteritis: Report of a large study and review of literature. Acta Ophthalmol Scand 2002;80: 355-367.
- Hayreh SS, Zimmerman B. Visual deterioration in giant cell arteritis patients while on high doses of corticosteroid therapy. Ophthalmology 2003;110:1204-15.
- Costello F, Zimmerman B, Hayreh SS. Thrombocytosis in giant cell arteritis: Diagnostic importance. Submitted for publication.
- Hayreh SS, Zimmerman B. Management of giant cell arteritis: Our 27-Year Clinical Study; New Light on Old Controversies. Ophthalmologica 2003;217:239-59.
- Hayreh SS. Steroid therapy for visual loss in patients with giant-cell arteritis. Lancet 2000;355:1572-3.
- Miller A, Green M, Robinson D. Simple rule for calculating normal erythrocyte sedimentation rate. Br Med J 1983;286:266.
- Hunder GG, Bloch DA, Michel BA, et al.: The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum 1990;33:1122-1128.
- Hayreh SS. Anterior ischaemic optic neuropathy: Differentiation of arteritic from non-arteritic type and its management. Eye 1990;4:25-41.
- Hayreh SS: Anterior ischaemic optic neuropathy. II. Fundus on ophthalmoscopy and fluorescein angiography. Br J Ophthalmol 1974;58:964-80.
- Hayreh SS. Anterior ischemic optic neuropathy. Heidelberg: Springer-Verlag, 1975.
- Hayreh SS: Acute ischemic disorders of the optic nerve: Pathogenesis, Clinical Manifestations and Management. Ophthalmol Clin N Am 1996;9:407-42.
For a more detailed bibliography on the subject, please refer to the large volume of references given in the publications listed above.
Scholarly articles in PubMed by Dr. Hayreh on the subjects of GCA and AION