
INITIAL PRESENTATION
Chief Complaint: Right eye pain
History of Present Illness:
A 29-year-old Caucasian man was referred to the cornea service for evaluation of corneal thinning in the right eye (OD). He was seen one week prior in glaucoma clinic for management of neovascular glaucoma and was found to have inferonasal corneal thinning and neovascularization.
He reported worsening right eye pain with daily retro-orbital headaches for 2-3 months and declining vision over the same period. He denied fever, unintentional weight loss, malaise, joint pain, rash, changes in urine or stool habits, shortness of breath, or recent travel.
He had a complex ocular history (see below) resulting in evisceration of the left eye (OS). Therapeutic interventions were unfortunately limited by poor compliance, narcotic abuse, and aggression towards staff members.
Past Ocular History:
Past Medical History:
Medications:
Allergies:
Family History: :
Social History:
Review of Systems: Pertinent positives and negatives as detailed in the history of present illness. Otherwise negative.
OCULAR EXAMINATION
| OD | OS | |
|---|---|---|
| Lids/lashes | Normal | Normal |
| Conjunctiva/sclera | Corneal patch graft in place with underlying glaucoma tube well covered. Diffuse conjunctival injection, most prominent inferonasally | No exposure, no papillae/follicles, conformer in place |
| Cornea | Crescentic thinning from 2:00 to 6:00 involving the limbus extending 3mm centrally with transversing pannus and severe thinning (about 10% normal stromal thickness remains) in some areas associated with fluorescein pooling but no epithelial defect. 1+ inferior keratic precipitates | Anophthalmic |
| Anterior chamber | Superotemporal tube in good position and patent, no cell or flare | Anophthalmic |
| Iris | No neovascularization | Normal architecture |
| Lens | Trace nuclear sclerosis | Anophthalmic |
| Anterior Vitreous | Optically empty, no vitreous hemorrhage | Anophthalmic |
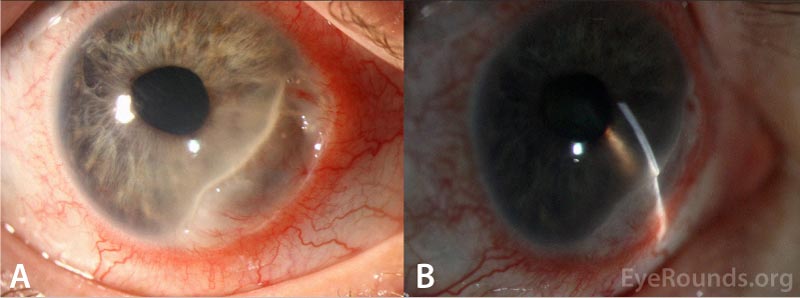
CLINICAL COURSE
Given the crescentic area of peripheral corneal thinning associated with pain, top differential diagnoses included peripheral ulcerative keratitis (PUK) and Mooren's ulcer. Labs including ESR, CRP, ANA, RF, anti-CCP, syphilis antibodies, QuantiFERON gold, angiotensin-converting enzyme (ACE), lysozyme, ANCA, hepatitis C RNA, and HIV were obtained to determine possible underlying infectious or autoimmune causes. Based on the patient's clinical presentation and a negative work-up for underlying systemic diseases, a diagnosis of Mooren's ulcer was made.
The patient's management and clinical course were complicated by a tenuous social situation and poor follow-up. There was hesitancy to start aggressive topical steroids due to the concern for progressive collagen destruction with unmonitored use given patient refusal to follow-up regularly. Topical medroxyprogesterone was also not possible due to the cost of this compounded medication.ion).
The decision was made to prescribe a single bottle of topical steroids (Pred Forte) without refills with the goal of improving the patient's pain and strengthening the therapeutic relationship. Prophylactic moxifloxacin drops QID and oral doxycycline 100 mg BID and vitamin C 1,000 mg daily were prescribed as well to promote collagen formation and reduce the risk of further stromal lysis.
The patient returned 4 months later with worsening eye pain. He had not started the recommended prescriptions. His visual acuity had decreased to light perception. The crescentic area of ulceration had progressed, now involving 12:30 to 9:00 with worsening focal areas of thinning superonasally (Figure 2). Seidel test remained negative.
Given poor compliance and progressive inflammation, the decision was made to proceed with resection of the adjacent conjunctiva (to halt delivery of pro-inflammatory agents to the ulcer bed) and application of cyanoacrylate glue in the operating room. Intraoperative examination demonstrating a severe overhanging edge of the ulceration is demonstrated in Video 1. A bandage contact lens was not placed due his history of poor compliance with antibiotic drops and concern that subsequent removal would be difficult in the clinic.

Mooren's Ulcer from University of Iowa Ophthalmology on Vimeo.
Post-operatively, the patient reported significant improvement in pain. On examination there was decreased conjunctival injection and stable severe thinning (Figure 3). It was difficult to ascertain whether the cyanoacrylate glue was still in place. Topical prednisolone and moxifloxacin were continued. His management is ongoing at time of publication.
DIAGNOSIS: Mooren's ulcer
DISCUSSION
Mooren's ulcer is a markedly painful ulcerative keratitis that can be rapidly progressive, relentless, and resistant to therapeutic intervention. By definition, Mooren's ulcer is not associated with underlying infectious or systemic autoimmune etiologies [1], and is therefore a diagnosis of exclusion.
Due to the often-intractable nature of this pathology, a significant proportion of the corneal stroma is destroyed and can frequently result in perforation of the remaining fibrovascular membrane [2]. Response to management is typically poor with visually debilitating outcomes.
Etiology/Epidemiology
Owing to the rarity of the Mooren's ulcer, clinically significant comparisons between studies and accurate estimations of its true prevalence are challenging to ascertain. Strong epidemiologic patterns and clearly defined clinical subtypes are not concretely established for patients with Mooren's ulcer, though there does appear to be a regional influence, as described in the following studies.
The first published report was in 1849, but the disease was not officially termed Mooren's ulcer until 1867 [3]. Several epidemiological studies have sought to characterize and sub-classify Mooren's ulcer. Wood and Kaufman initially reported 9 cases of Mooren's ulcer in 1971, classifying the disease into "limited" and "atypical/malignant" types [4]. The former was thought to be a benign, unilateral variety found in older patients with mild symptoms and good response to surgical treatment. The latter was characterized by more severe symptoms and poorer response to therapy, most often found bilaterally and in younger patients. Decades later in 1990, Lewallen and Courtright published contrasting findings, demonstrating that more severe, bilateral disease occurred more often in older patients compared to those under 35 years (43% vs 33.3%) [5], and occurring twice as often in white patients compared to black patients. However, several aspects (i.e. study period > 85 years, poor documentation, poor follow-up, changes in diagnostic criteria over time), limit the validity of this epidemiologic study and its findings.
Since then, additional studies, including those by Watson et al. [6] and numerous case series reported from South India [7], China [8], and Nigeria [9], have been conducted to further characterize this disease. One study in particular demonstrated a potential difference in West African populations with higher prevalence in younger males around 20-40 and presenting with a rapidly progressive bilateral course [10].
Pathophysiology
The precise mechanism underlying Mooren's ulcer is uncertain, but seems to be an immune response to corneal antigens. Prior reports demonstrate the presence of inflammatory cells, immunoglobulin, and elevated HLA class 2 molecules within the affected tissue, implicating both cell-mediated and humoral immunity in the pathogenesis [11]. Molecular mimicry may occur after specific provocations, including surgery, hepatitis C infection (as noted in our patient), hookworm infections, or trauma [12]. Certain individuals may carry a genetic predisposition, as HLA-DR17 positive individuals have been shown to have a higher relative risk of developing Mooren's ulcer (RR 2.54; 95% CI 1.16 to 5.57; p=0.03) [13].
Signs/Symptoms
Patients most commonly report severe pain, redness, tearing, photophobia, and decreased vision. Approximately 1/3 of cases present bilaterally [14]. On exam there is a crescent shaped ulceration in the peripheral cornea concentric to the limbus, often in the interpalpebral zone. There may be adjacent conjunctival and episcleral inflammation, but the sclera itself is spared. Occasionally Mooren's ulcer is also associated with iritis [2].
With ongoing inflammation, the ulcer progresses circumferentially as well as centrally [1], often with a linear epithelial defect at its leading edge and significant stromal loss. Careful exploration will reveal the true depth of destruction, which may be much more than is initially apparent at the slit lamp. The bed becomes vascularized and can have such severe loss of stroma as to only leave a fibrovascular membrane.
Testing/Laboratory work-up
Mooren's ulcer is a diagnosis of exclusion. It must be differentiated from other painful peripheral ulcerations including infectious keratitis and peripheral ulcerative keratitis (PUK), the latter of which is a manifestation of underlying autoimmune disease. A thorough history and review of systems should be performed. Initial laboratory work-up may include a complete blood count (CBC), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), rheumatoid factor (RF), antinuclear antibody (ANA), antineutrophil cytoplasmic antibodies (c-ANCA and p-ANCA), liver function panel (LFT), hepatitis panel, syphilis screening (venereal disease research laboratory test (VDLR) or fluorescent treponemal antibody absorption test (FTA-ABS)), tuberculosis screening (QuantiFERON gold or protein purified derivative (PPD)), renal function (UA, BUN, and creatinine), and serum protein electrophoresis (SPEP). Culture of the ulcer can be performed to rule out microbial keratitis. Additional studies, such as a chest x-ray, skin biopsy, or sacro-iliac joint imaging, may be carried out depending on the level of suspicion.
The presence of pain helps distinguish Mooren's ulcer from other painless causes of peripheral corneal thinning such as Terrien's marginal degeneration, pellucid marginal degeneration, and senile furrow degenera
Treatment/Management/Guidelines
Management of Mooren's ulcer follows a stepwise fashion and should include:
Unfortunately, response to treatment is often poor and the final outcome can be visually debilitating.
Initial treatment involves local immunosuppression with aggressive topical steroids (usually every hour) along with prophylactic antibiotic drops. Inflammation must be monitored closely with examinations every few days initially. Topical steroids are tapered based on clinical response.
Concurrently with local immunosuppression, additional therapy to promote collagen healing, such as topical medroxyprogesterone, oral tetracyclines (doxycycline), and oral vitamin C, may also be beneficial. Other treatments that have been reported to be effective in refractory cases include topical cyclosporine 1% [15] and topical interferon alpha-2b [16].
Subsequent systemic immunosuppression may also be necessary and should be implemented in conjunction with an internist or rheumatologist to monitor for possible therapeutic side effects. Commonly used agents include systemic corticosteroids (1.0-1.5 mg/kg body weight/day), methotrexate, cyclophosphamide, and azathioprine.
If the above interventions are inadequate, surgical resection of the conjunctiva should be considered. The conjunctiva up to 2 clock hours on either side of the ulcer and 3-4 mm posterior to the limbus should be excised with the goal of reducing delivery of inflammatory agents to the cornea [17]. Cryotherapy of the conjunctiva may achieve a similar effect [18].
Even with the above interventions, the risk of severe corneal thinning and perforation is high, and is estimated to be approximately 30% in several studies [19, 20]. Focal areas of thinning are often temporized with application of cyanoacrylate glue, whereas larger defects may necessitate a patch graft or lamellar keratoplasty [21]. Corneal transplantation in the setting of acute inflammation has an extremely poor prognosis.
Once the inflammation has subsided, the goal of management shifts to preservation and rehabilitation. Patients with visually significant sequelae such as irregular astigmatism and scarring may benefit from scleral contact lenses. We recommend that this be done only in conjunction with an optometrist highly experienced in fitting specialty contact lenses for patients with corneal pathology. Areas of tectonic weakness may be stabilized surgically with lamellar or full-thickness keratoplasty, with careful attention paid to peri-operative immunosuppression to minimize the risk of recurrent inflammation. However, some patients may be best served by no further surgical intervention and careful activity restrictions to minimize risk of perforation with minor trauma.
ETIOLOGY/EPIDEMIOLOGY
|
DIAGNOSIS
|
SYMPTOMS
|
TREATMENT/MANAGEMENT
|
Silverman JIM, Yu CY, Kam Y, Ling J. Mooren's Ulcer: A Diagnosis of Exclusion. EyeRounds.org. January 20, 2022. Available from https://EyeRounds.org/cases/316-moorens-ulcer.htm

Ophthalmic Atlas Images by EyeRounds.org, The University of Iowa are licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License.