
The University of Iowa
Department of Ophthalmology and Visual Sciences
April 26, 2013
Age-Related Macular Degeneration (AMD) is the most common cause of blindness in the Western world. Currently it is estimated that about 2 million Americans are affected by AMD and by the year 2020 it is projected to be closer to 3 million as life expectancy increases and the aging population expands.[1] This is a critical issue involving a significant proportion of the elderly, as vision is essential for maintaining independence and quality of life. A decline in visual acuity increases the risk for falls and depression, both of which are significant sources of morbidity and mortality in the geriatric population.
Risk factors for AMD include increasing age, smoking, family history, and race. AMD is rarely found in individuals younger than 50 and has been reported in 30% of Americans over the age of 85.[1] A family history of AMD does confer an increased risk, which is typically multifactorial in nature. Polymorphisms in the complement factor H (CFH) gene, HTRA/ARMS2, and at other loci have been shown to confer an increased risk of AMD among Caucasians.[2,3] CFH acts as an inhibitor of complement, and this polymorphism is thought to weaken its ability to down-regulate complement activity. This permits continued inflammation and pathogenic degeneration of the macula.[2] AMD is most common in Caucasians; Hispanics and Asians have an increased prevalence compared to African Americans.[ 1] Smoking is the most significant modifiable risk factor and cessation should be encouraged at each visit to prevent continued vision loss. While current smokers are 2-4 times more likely to be affected by AMD than nonsmokers of the same age, those who are smoke-free for 20 years may decrease their risk of AMD to that of a non-smoker.[4] Other speculated risk factors for AMD include obesity, hypertension, hyperlipidemia, hyperopia, light iris color, female gender, cardiovascular disease, and nutritional deficiencies.[2,3]
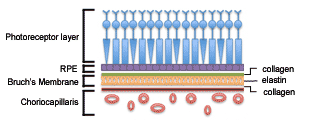
The retina is the light-sensing portion of the eye, and is composed of different cell types arranged in layers. The cell layers are described as inner and outer laminae in regards to where they are located relative to the center of the eye. The photoreceptors comprise the outermost layer of the retina and are located above the retinal pigment epithelium (RPE). The RPE is responsible for maintaining the extracellular matrix and the activity of the photoreceptors. Beneath the RPE is Bruch’s membrane, which maintains the blood-ocular barrier. Bruch’s membrane is composed of a central elastin core sandwiched between layers of collagen. The choriocapillaris, the innermost layer of the choroid, lies directly beneath Bruch’s membrane and supplies most of the blood or oxygen to the outer one-third of the retina. It also helps remove waste created from the photoreceptors and RPE activity. The short posterior ciliary arteries supply the choriocapillaris. Together, the RPE, Bruch’s membrane, and choriocapillaris (Figure 1) maintain the health and function of the overlying photoreceptors and thus, support vision.[5]
The central retinal artery supplies blood to the inner two-thirds of the retina and enters the eye with the optic nerve. These are the artery branches visible on the fundus exam. After entering the eye the artery branches into a superior and an inferior arch that surrounds the macula.
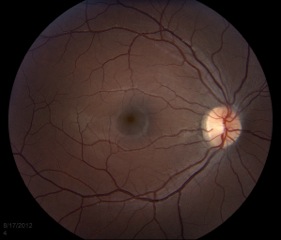
The macula is the area of the retina that is responsible for central vision. It is used to do things such as threading a needle, reading fine print, recognizing faces, and watching TV. In a normal exam, the fovea, located at the center of the macula, appears to be darker than the surrounding retina (Figure 2).
AMD can be classified as “dry” (a.k.a. atrophic or non-neovascular) or “wet” (a.k.a. neovascular or exudative) form. Most cases of AMD are “dry” (80%) while the remaining 20% of cases are considered “wet.” However, “wet” AMD accounts for 90% of cases with advanced stage disease and severe vision loss.[5] The symptoms and treatments differ between classes and therefore will be described separately in the following sections.
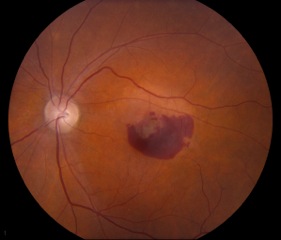
Dry AMD is identified on exam by diffuse or localized drusen and geographic atrophy. Geographic atrophy can be defined as a loss of RPE and photoreceptors that appear as a well-demarcated yellowish area often with choroidal vessels more evident on exam and symptomatically produce scotomas. Wet AMD, by contrast, features newly-formed vessels known as choroidal neovascular membranes (CNV). Similar to neovascularization that occurs in diabetic retinopathy, CNVs are immature and leaky. Consequences of neovascularization include subretinal fluid, lipid deposition, hemorrhage, pigment epithelial detachment (in which fluid separates the RPE from Bruch’s membrane), and fibrotic scarring all of which cause vision changes or loss.
The Age-Related Eye Disease Study (AREDS) defined categories based on the exam findings of drusen, atrophy, and neovascularization; these categories are defined below.[3,6]
When estimating the size of funduscopic findings it is helpful to know that the average optic disc (optic nerve head) is about 1500 microns in diameter and a vein width near the disc is about 120-150 microns. Therefore, a druse with a diameter the same width as a vein near the disc is considered “large.”
The macula is specifically affected in AMD and hence there is a loss of central and fine detailed vision. The surrounding retina is mostly spared and therefore peripheral vision is generally intact.
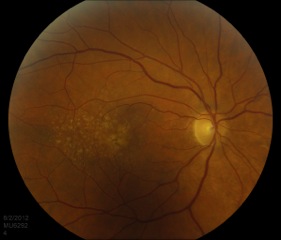
Drusen are the clinical hallmarks of AMD and are typically the earliest exam finding. They are deposits, comprised of extracellular matrix and inflammatory components (e.g. complement) located in Bruch’s membrane. Drusen appear pale and yellow and they can be categorized as “hard” or “soft” based on appearance. Hard drusen are small, well-demarcated lesions and can be seen in the healthy, aging population. Soft drusen are large and appear ‘cotton-ball’ like (Figure 3). They are generally pathologic and are not found in young, healthy populations.[7]
The pathogenesis of AMD is not completely clear. Scientists feel that AMD is likely a pathological extension of normal aging that occurs within the eye. It does not appear that there is one mechanism that causes AMD, but that it stems from a collection of complex processes that combine to create the disease state with age. However, it is clear that both genetics and the environment play a role in the pathogenesis.
Bruch’s membrane plays a critical role in the pathogenesis of AMD. In young, healthy eyes, Bruch’s membrane functions as a structural support that is permeable to fluid and small molecules, such as oxygen and glucose. It also acts as a barrier against neovascularization by containing molecules in the elastin layer that are anti-angiogenic. As the RPE functions on a daily basis it creates vascular endothelial growth factor (VEGF) that can pass through Bruch’s membrane to the choriocapillaris. However, the anti-angiogenic molecules within Bruch’s membrane prevent growth of neovascular capillaries from the choroid thus helping prevent wet AMD.
As we age, Bruch’s membrane tends to accumulate debris in the elastin lamina and also drusen between the collagen layer and RPE basal lamina. This debris accumulation causes a reduction in the permeability of Bruch’s membrane. This will hinder the pumping of waste from inside to outside of the eye by the RPE and may cause pigment epithelial detachments. In addition, atrophy, as seen in dry AMD, is thought to be caused by excess material deposited in Bruch’s, thus hindering the connection to the choroid for vascular supply and removal of waste.[8]
Aging is also associated with thinning and breakdown of the central elastin layer within Bruch’s membrane. Thinning of this layer increases the risk of neovascularization as it reduces the number of bound anti-angiogenic proteins. In addition, elastin breakdown products (elastin-derived proteins) are themselves angiogenic. Therefore, breakdown of elastin in Bruch’s membrane not only causes a reduction in the barrier to neovascularization, but also stimulates vessel growth.
Dry macular degeneration may initially be asymptomatic, or may present as blurred vision, decreased contrast perception, difficulty adjusting from bright to dim lighting, or the need for brighter lighting or more magnification when reading. Central scotomas (visual field defects) are present in the advanced stage of non-neovascular age-related macular degeneration. These symptoms usually progress slowly, over months to years.[2,3]
Wet macular degeneration may have symptoms of metamorphopsia (straight, linear objects appear wavy or distorted) or central scotomas. Symptoms usually present as a sudden decline in vision and progress rapidly over days to weeks.[2,3]
The Amsler grid is a pictorial tool used to identify the presence of metamorphopsia or scotomas. Dry AMD may or may not have changes in visual acuity, while wet AMD is more likely to cause sudden changes in visual acuity. However, both types can have changes on the Amsler grid. The grid can be rechecked and recorded at each visit to help determine progression. A grid should also be given to each patient for detection of changes at home, with instructions to check each eye separately. The earlier the changes are detected, the higher the likelihood that a CNV can be detected at a treatable stage.[3]
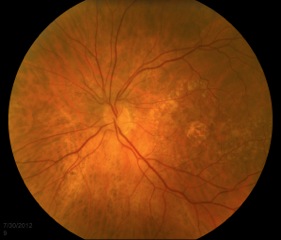
Small, hard drusen and larger soft drusen within the macula are characteristic of dry AMD. Atrophy of the RPE may also be present and appears as a well-demarcated area of yellowing within the retina with easy visualization of the choroidal vessels[3] (Figure 4). Drusen are also present in cases of wet AMD, but CNV are inherent. Hemorrhage or subretinal fluid may also be visible (figure 5). CNV appear as a well-demarcated graying of the retina.[3]
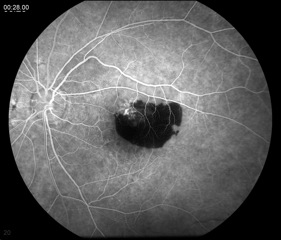
This test entails the injection of fluorescein dye intravenously and the use of special filters on a fundus camera to capture the fluorescent properties of the dye. Serial photos are taken over several minutes as the choroid and retina are supplied with blood marked by the dye. An FA can demonstrate slow filling of the choroid or retinal vessels (increased transit time), window defects caused by loss of RPE (hyperfluorescence), blood vessel leakage (hyperfluorescence), or blocking lesions (hypofluorescence).[9] An FA is indicated when an AMD patient complains of new metamorphopsia, unexplained blurred vision, and/or when clinical exam reveals elevation of the retina, subretinal hemorrhage, or hard exudates.[5] The results from the FA are mostly used to guide treatment, with either lasers or intravitreal injections, and less so for diagnosis.
A patient with dry AMD and atrophy will have a window defect on FA. This window defect is from the RPE atrophy that allows the fluorescence from perfusion of the choroid to show through, without the RPE “blocking” the fluorescence from the camera. Therefore, this appears as an area of hyperfluorescence. Drusen may appear as areas of punctate hyperfluorescence.[3]
Wet AMD may show leakage from CNV (hyperfluorescence) and/or blocking lesions from a subretinal hemorrhage (Figure 6). Subretinal hemorrhage blocks the fluorescence of the choroid from the camera, thus appearing black or hypofluorescent.[3]
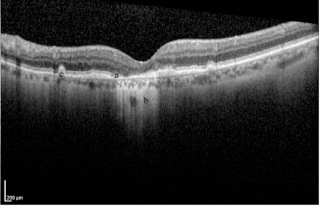
Geographic atrophy is demonstrated on OCT as an absence of the RPE layer and increased signal deep to the RPE relative to adjacent areas where the RPE is intact. Drusen may be visible as elevations at the level of the RPE[3] (Figure 7). OCT is used to determine if there is blood or fluid beneath the retina (subretinal) or within the retinal layers (intraretinal). Fluid appears as black gaps or elevations and is indicative of wet AMD. OCT is often used to determine if retreatment with anti-VEGF injections is necessary or in cases where the fluorescein angiogram is equivocal.[3]
There is currently no treatment for dry AMD; however, recent attention has been given to AREDS vitamins, so named because of the formulation used in the AREDS trial. It is recommended that patients with intermediate or advanced stages supplement with high doses of antioxidants and specific vitamins (vitamin C, vitamin E, beta-carotene, zinc, and copper) to decrease the risk for conversion to wet AMD.[6] However, current or former smokers are discouraged from taking high doses of beta-carotene due to an increased risk of lung cancer.[6] There is a supplement formulation available for current smokers or those who have smoked in the last 10-15 years that substitutes lutein for beta-carotene. Patients with rare drusen, mild AMD, or a strong family history can supplement with lower doses of vitamins and antioxidants, such as those found in over the counter multi-vitamin preparations. Patients should be encouraged to use the Amsler grid every day and counseled on low vision aids as needed.[3] Smoking cessation should also be discussed and assistance with quitting provided if necessary.[5]
Anti-VEGF intravitreal injections have become the first line of treatment for all forms and locations of CNV, but especially for treating subfoveal locations, as these injections are less likely than laser photocoagulation to cause damage to the retina and loss of central vision. Anti-VEGF agents include pegaptanib sodium (Macugen), bevacizumab (Avastin), ranibizumab (Lucentis), and aflibercept (Eylea).[2,3] Currently, pegaptanib sodium, ranibizumab, and afibercept are FDA-approved for treating wet AMD. It was previously thought that ranibizumab was more effective than bevacizumab, but a pivotal treatment trial demonstrated similar efficacy and safety between the two.[10]
Focal laser photocoagulation is an effective neovascularization treatment that now has limited utility in the anti-VEGF era. Photocoagulation can result in permanent visual field defects as it essentially involves burning CNV membranes and surrounding retina. Therefore, it is not recommended for CNV located within the macula.[2,3] Some physicians choose to continue using this therapy in extrafoveal lesions such as in peripapillary CNVs (those that lie within one disc diameter of the nerve head). However, anti-VEGF treatments, such as bevacizumab, have been shown to be as effective in extrafoveal lesions and may actually be the better treatment option.[11]
Photodynamic therapy (PDT) consists of injecting a dye (verteporfin) intravenously that tends to concentrate in new blood vessels and is activated by a laser beam. Activation causes localized thrombosis and resolution of the CNV, but does not improve vision loss, as it causes damage to the retina.[2] PDT is FDA-approved for wet AMD, but has also been largely replaced by the anti-VEGF treatments. However, it may be effective when used in combination with anti-VEGF agents for patients who do not respond or no longer respond to anti-VEGF injections alone.[12]
Low vision aids are strongly recommended for patients with macular degeneration who struggle with activities of daily living and smoking cessation should be discussed to reduce further vision loss.[5]
The Age-Related Eye Disease Study was designed to address the following question: “Does zinc and antioxidant supplementation slow vision loss associated with increasing age, such as AMD and cataracts?” It was composed of a clinical trial for supplementation in AMD and another trial for cataracts. For AMD, each participant was staged using the category system mentioned above (see “Classification”). They were then randomly assigned to one of four daily oral tablet supplementation groups (zinc alone, antioxidants alone, zinc plus antioxidants, or placebo). Overall, they found that those patients with intermediate AMD or those with advanced AMD in only one eye had a 25% lower risk of progressing to advanced AMD in one or both eyes, respectively, when treated with zinc plus antioxidants. Patients categorized as having no or early AMD did not show a benefit with the high dose supplementation.
Smoking is the most important modifiable risk factor to prevent/reduce morbidity from AMD, and cessation should be emphasized in these patients. However, if they choose to continue or if they have smoked in the last 10-15 years they should not have beta-carotene supplementation as it increases the risk of lung cancer. There is a special AREDS vitamin formulation for these individuals.
There is preliminary evidence that omega-3 fatty acids or fish oil and lutein supplements may be of benefit to reduce the incidence of wet AMD.* This is currently being studied as a clinical trial, AREDS2, which is projected to have results announced in 2013.
Haugsdal J, Sohn E. Age-related macular degeneration: from one medical student to another. EyeRounds.org. Apr 26, 2013; Available from https://eyerounds.org/tutorials/AMD-medical-student/