Chief Complaint: New floaters in both eyes.
History of Present Illness: A 60-year-old gentleman with a history of hepatitis C virus (HCV) infection presents with new floaters in both eyes. He started treatment for HCV with peginterferon and ribavirin 6 weeks prior to this visit.
Four weeks prior to this visit, the patient was seen in our eye clinic at the University of Iowa for a history of floaters and a few episodes of prisms of light in both eyes lasting for 5 to 10 seconds. His examination at that time, which included a detailed funduscopic examination, was unremarkable.
The patient was instructed to return to clinic in 2 weeks for a reevaluation, but returned in 4 weeks instead. At this follow up visit he reported that the floaters had resolved.
Past Ocular History: Presbyopia, no previous ocular surgeries, no trauma
Medical History: Hepatitis C virus, tobacco use
Medications: Erythropoetin, peginterferon, ribavirin
Allergies: No known drug allergies
Family and Social History: Non-contributory
Slit Lamp Examination:
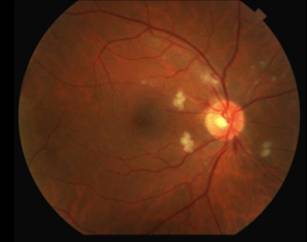 |
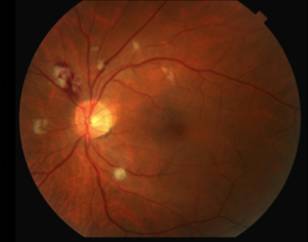 |
The differential diagnosis for diffuse CWS surrounding the optic nerves in both eyes (Figure 1) includes: interferon-associated retinopathy, hypertensive retinopathy, retinopathy associated with leukemia/lymphoma, ocular ischemia, Purtcher's retinopathy and HIV retinopathy. Given that the patient had recently started interferon therapy, was HIV negative, had a normal blood pressure and blood cell count and had no risk factors for ocular ischemia or Purtcher's retinopathy, the most likely diagnosis was interferon-associated retinopathy.
Hepatitis C virus (HCV) primarily affects the liver. However, patients infected with HCV are often asymptomatic, and the disease frequently goes unnoticed until severe scarring of the liver (cirrhosis) occurs. There is no cure for HCV; however, various medications can be used to decrease the viral load. A common treatment regimen includes a combination of interferon and ribavirin (Schulman et. al. 2001).
Many reports have discussed the ocular complications associated with interferon use as an antiviral or antiangiogenic agent. Ikebe first reported this condition in a 39-year-old gentleman who developed retinopathy after administration of intravenous interferon (Ikebe et. al. 1990). Other complications associated with interferon include ischemic optic neuropathy, subconjunctival hemorrhage, retinal hemorrhage, combined choroidal and retinal perfusion deficits and cystoid macular edema (Guyer et. al. 1993, Schulman et. al. 2001, Tokai et. al. 2001). Interferon associated retinopathy occurs in 19% to 69% of adults on interferon therapy (Narkewicz et. al. 2010, Schulman et. al. 2001).
Interferon-associated retinopathy often presents with cotton wool spots, retinal hemorrhages and other retinal microvascular irregularities (Esmaeli et. al. 2001). These changes occur most notably around the optic nerve head and in the posterior pole (Schulman et. al. 2001). The retinopathy typically presents 3 to 5 months after treatment begins; however, as in our case, it can present as early as 2 to 6 weeks into the treatment (Kadayifcilar et. al. 1999, Okuse et. al. 2006).
Frequent monitoring of patients on interferon is important. Patients with normal exams should be followed every 4 to 6 months while those with retinopathy should be followed more frequently while on treatment. Fortunately, the ocular findings of interferon-associated retinopathy appear to reverse with cessation of treatment, including CME in one case (Tokai et. al. 2001). When the cotton wool spots encroach upon the macula or when CME is present, it is important for the ophthalmologist to work with the internal medicine team and consider terminating interferon treatment (Meltzer et. al. 2008).
Our patient's HCV disease was advanced, and the hepatologist felt that the patient had a guarded prognosis despite interferon treatment. The patient's initial hepatitis C viral load was very high (>1 million IU/mL). However, after only 6 weeks of treatment (around the same time that the retinopathy was noted), his viral load became undetectable. We were concerned about visual prognosis as the CWS were close to the macula, but the hepatology service, understandably, had hoped to continue treatment given its success.
Extensive discussions took place between the patient, his family and the hepatology and ophthalmology services. An attempt was made to weigh the potential risk to the eye against the significant benefit of decreased viral load and activity of HCV while on peginterferon. After careful deliberation, the patient decided to continue with the HCV treatment for now, considering his great response thus far and the likelihood that he would not need to be on the treatment for an extended period of time. The ophthalmology service agreed to closely monitor the patient for CME and other signs of worsening interferon-associated retinopathy.
Fortunately on examination 2 weeks later (8 weeks into interferon therapy) the interferon retinopathy was stable if not a bit better (Figure 2).
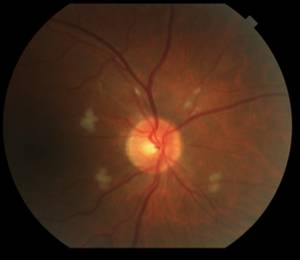 |
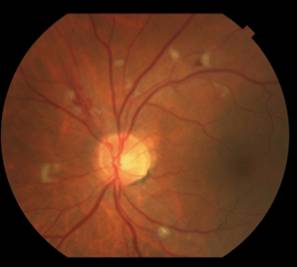 |
Our ophthalmology service will continue to monitor the retinal exam every 4 to 8 weeks and communicate closely with the internal medicine service.
Diagnosis: Interferon-associated Retinopathy
Epidemiology:
|
Signs:
|
Symptoms:
|
Treatment:
|
Esmaeli, B., Koller, C., Papadopoulos, N., Romaguera, J. Interferon-induced Retinopathy in Asymptomatic Cancer Patients. Ophthalmology 2001;108:858-860.
Guyer, DR., Tiedeman, J., Yannuzzi, L., Slakter, J., Parke, D., Kelley, J., Tang, R., Marmor, M., Abrams, G., Miller, J., Gragoudas, E. Interferon-Associated Retinopathy. Arch of Ophthalmol 1993;111:350-356.
Ikebe, T., Nakatsuka, K., Goto, M. A Case of Retinopathy Induced by Intravenous Administration of Interferon. Folia Ophthalmol 1990;41:2291-2296.
Kadayifcilar, S., Boyacioglu, S., Kart, H., Gursoy, M., Aydin, P. Ocular Complications with High Dose Interferon-alfa in Chronic Active Hepatitis. Eye 1999;13:241-246.
Meltzer, D. Interferon Retinopathy: A Side Effect from the Treatment of Hepatitis C. Optometry 2008;79 320-321.
Narkewicz, MR., Rosenthal, P., Schwarz, KB., Drack, A., Margolis, T., Repka, MX. Ophthalmologic Complications in Children with Chronic Hepatitis C Treated with Pegylated Interferon. Journal of Pediatric Gastroenterology and Nutrition 2010; Published ahead of print. (PMID: 20512062)
Okuse, C., Yotsuyanagi, H., Nagase, Y., Kobayashi, Y., Yasuda, K., Koike, K., Iino, S., Suzuki, M., Itoh, F. Risk Factors for Retinopathy Associated with Interferon alpha-2b and Ribavirin Combination Therapy in Patients with Chronic Hepatitis C. World J Gastroenterol 2006;12:3756-3759.
Schulman, JA., Liang, C., Kooragayala, LM., King J. Posterior Segment Complications in Patients with Hepatitis C Treated with Interferon and Ribavirin. Ophthalmology 2003;110:437-41.
Tokai, R., Ikeda, T., Miyaura, T., Sato, K. Interferon-Associated Retinopathy and Cystoid Macular Edema. Arch Ophthalmol 2001;119:1077-1079.
Wes A, Hong ES, Oetting TA. Interferon-Associated Retinopathy: Communicating with Internal Medicine. EyeRounds.org. July 26, 2010; Available from: http://www.EyeRounds.org/cases/116-Interferon-Retinopathy.htm.

Ophthalmic Atlas Images by EyeRounds.org, The University of Iowa are licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License.