Decreased vision
The patient is a 61-year-old male who presents with worsening vision in both eyes. Two years prior, at the time of his last dilated exam, he was 20/25 in both eyes. Since that time, he has noticed a gradual decline in vision in both eyes. He has poorly controlled diabetes mellitus type II with a recent hemoglobin A1c of 8.0%. His diabetes is treated with oral medications. He denies any sudden loss of vision, flashing lights, new floaters, or headaches.
The patient was diagnosed with clinically significant macular edema (CSME) in the setting of severe non-proliferative diabetic retinopathy in both eyes and was treated with focal laser in the left eye followed by the right eye. He did not notice an improvement in vision after the laser treatment and his visual acuity was essentially unchanged at follow-up. He was given sub-tenon's Kenalog® (triamcinolone acetonide) (STK) OD and intra-vitreal Avastin® (bevacizumab) OS for persistent macular edema. 1 month later his vision and diabetic macular edema (DME) had improved in the right eye, but worsened in the left eye. As a result of the improvement in the right eye, STK was given in the left eye which yielded a reduction in macular thickness on OCT and improvement in visual acuity. Given his favorable response to steroid treatment, he received 1 more dose of STK to both eyes before developing elevated intraocular pressure. Unfortunately, the elevated intraocular pressure was not responsive to topical anti-hypertensive medications and further treatment with steroids was abandoned 2 years after initial presentation.
The patient had persistent DME with visual acuity of 20/50 OD and 20/100 OS. Also over this course, he was found to have proliferative diabetic retinopathy OD that was treated with pan-retinal photocoagulation. After cessation of steroids, he received regular intravitreal injections with Avastin which failed to impact the macular edema. Due to the lack of response to conventional therapy with persistent macular edema and more than usual venous dilation, screening for underlying metabolic disease or condition causing hyperviscosity which could potentially be contributing to his retinal pathology was arranged. Pertinent results included:
He was referred to an endocrinologist for further investigation of his anemia and hyperproteinemia. Further testing revealed:
The patient's anemia combined with serum protein electrophoresis showing an IgM monoclonal gammopathy and elevated serum viscosity led to the diagnosis of hyperviscosity syndrome due to Waldenstrom Macroglobulinemia. The patient was referred to the hematology service for further treatment recommendations.
He was initially treated with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) chemotherapy upon diagnosis which produced little reduction in IgM levels. Multiple second and third line chemotherapy regimens were attempted; however, his IgM levels remained elevated between 6500-7000 mg/dl. Next, he began scheduled plasmapheresis and IVIG therapy every 4 weeks. With this treatment regimen, his IgM level was consistently maintained in the 1000-2000 range and the macular edema in both eyes began to resolve (Fig 4). Despite not responding initially to standard chemotherapy treatments, he has been able to maintain a monthly schedule of plasmapheresis and IVIG for over four years. His macular edema also requires intermittent intravitreal injections of triamcinolone to control. As a result, his visual acuity has improved to a baseline of 20/60 in both eyes with minimal residual cystoid macular edema (CME) (Fig 5).
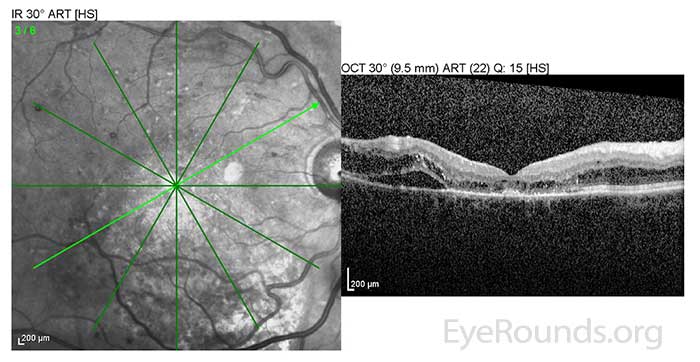 OD: Resolving macular detachment and surrounding CME. |
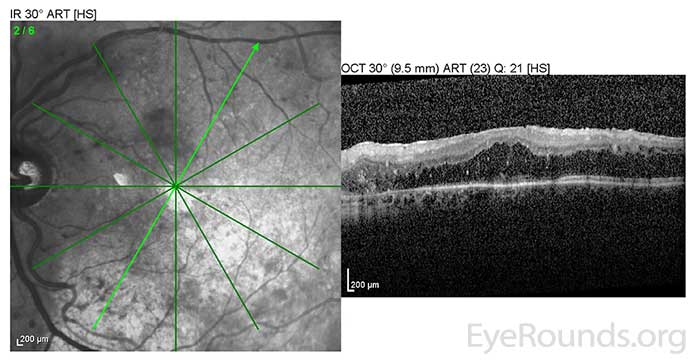 OS: Resolved neurosensory retinal detachment with diffuse overlying CME. |
Waldenstrom macroglobulinemia (WM) is a lymphoplasmacytic lymphoma characterized by malignant B cells and the accumulation of IgM monoclonal protein in the blood. While no single cause has been found, a mutation in the MYD88 gene is observed in over 90% of patients (1). This gene encodes for a protein involved in the toll-like receptor and interleukin 1 signaling pathways. The most common mutation seen in WM patients, MYD88 L265P, is thought to lead to constitutive expression of the anti-apoptotic transcription factor NF-kB, allowing the abnormal IgM producing B cells to survive (2). The accumulation of IgM protein leads to both blood hyperviscosity and high intravascular osmotic pressure (3). The presence of a large volume of positively charged IgM molecules interacts strongly with red blood cells contributing to blood hyperviscosity.
Waldenstrom macroglobulinemia is a rare disease with an incidence of 3 cases per million people per year in the US. This results in roughly 1,000 to 1,500 new diagnoses each year. The major risk factors are male gender, Caucasian race, and age over 60 (4). Fundoscopic abnormalities are seen in approximately 30-40% of patients with WM. Common exam findings include bilateral retinal vein engorgement, lipid exudates, central and branch retinal vein thrombosis, and flame-shaped hemorrhages, which typically manifest once serum viscosity rises to near 4.0 cp (5). A less common ocular finding is the accumulation of subretinal fluid, as seen in this patient. Several studies have documented the presence of immunoglobulins in the subretinal space of patients with WM and multiple myeloma. Although the exact mechanism for this finding is unknown, multiple theories have been proposed. Klaassen et al suggested that venous stasis retinopathy caused by serum hyperviscosity leads to hypoxia of the retinal vascular endothelial cells. As a result, there is breakdown of the blood-retinal barrier allowing passage of IgM into the subretinal space (5). This mechanism could easily be exacerbated by comorbid conditions which produce an ischemic state in the retina, particularly diabetic retinopathy, which was documented in this patient more than 20 years prior to the diagnosis of WM.
In addition to intraocular manifestations, patients with Waldenstrom macroglobulinemia commonly develop lymphadenopathy (25%), hepatomegaly (24%), and splenomegaly (19%) (5). In a study assessing 217 patients with WM, the most common symptoms leading to diagnosis were anemia (38%), B symptoms (23%), bleeding (23%), and neurological symptoms (22%) (5). Despite the systemic nature of the disease, WM is found incidentally in up to 27% of patients who do not express any signs or symptoms of the disease.
Patients with WM often have plasma IgM levels upwards of 9000 or more (normal 40-230) with significant serum hyperviscosity. Initial treatment of symptomatic patients should be targeted toward decreasing IgM through plasmapheresis. Studies have shown that plasmapheresis is effective at decreasing serum IgM between 35%-48% (7, 8). Rapid reduction of IgM levels will usually lead to relief of hyperviscosity syndrome complications including reversal of retinal vein engorgement (8). The patient in this case responded well to plasmapheresis and IVIG therapy with reduction of subretinal fluid, flame hemorrhages and vessel tortuosity. Although these patients are often anemic, red blood cell transfusions should not be done as the ensuing interaction of IgM with donor RBCs may increase serum viscosity.
Once the serum IgM level is controlled with a documented decrease in viscosity, most patients require maintenance with chemotherapy. For years, CHOP therapy was the preferred treatment regimen. Then, a 2009 study found that the addition of rituximab to CHOP in patients with WM increased the response rate from 60% to 91% and increased the time to treatment failure from 22 months to 63 months with no difference in toxicity (9). Although VEGF inhibitors may aid in reducing intraretinal and subretinal fluid accumulation seen in WM retinopathy, this option should only be considered in conjunction with treatments designed to remove the abnormal IgM complexes from serum.
The most recent recommendations favor treatment of WM with either DRC (dexamethasone, rituximab, and cyclophosphamide) or BR (bendamustine and rituximab) over the previous standard of care (R-CHOP) (6). This change is due to studies showing similar response rates in R-CHOP and DRC or BR, but increased progression-free survival and tolerability over R-CHOP (10).
This case represents a significant diagnostic challenge. Patients with severe nonproliferative and proliferative diabetic retinopathy commonly develop clinically significant macular edema. In the vast majority of cases, patients with a history of diabetic retinopathy would lead the examiner to the probable diagnosis of CSME due to uncontrolled diabetes. However, at what point should other, more atypical etiologies of persistent CSME be considered? In this patient, the lack of a normal response to well-established treatments like VEGF inhibitors and steroids and the significant bilateral venous dilation and tortuosity tipped off the examiner that there may be something else at play masquerading as a common complication of diabetic retinopathy. For this reason, it's important to keep other etiologies in mind, including paraproteinemias like WM and multiple myeloma (3).
EPIDEMIOLOGY
|
SIGNS
|
SYMPTOMS
|
Management
|
Flanary WE, Meirick TM, Boldt HC. Waldenstrom Macroglobulinema-Associated Retinopathy. EyeRounds.org. posted August 21, 2015; Available from: http://www.EyeRounds.org/cases/216-Waldenstrom-retinopathy.htm

Ophthalmic Atlas Images by EyeRounds.org, The University of Iowa are licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License.