Central Serous Chorioretinopathy
Central Serous Chorioretinopathy
INITIAL PRESENTATION
Chief Complaint
Blurry vision in the left eye
History of Present Illness
A 45-year-old man presented with blurry central vision in the left eye for a week. He works as a barber and noticed images in the left eye were blurry, distorted, and yellow-tinted while working. He first noticed the visual symptoms several days after starting oral steroids for arthritis in his wrist. He denied any associated peripheral vision loss, flashes of light, pain, redness, or photophobia. Of noted, he plays curling semi-professionally and reported heightened stress related to training for an upcoming tournament.
Past Ocular History
Past Medical History
- Arthritis
- Asthma
- Herniated lumbar disks
Medications
- Methylprednisolone 4 mg three times daily
- Epidural cortisone injections, once monthly
- Albuterol and fluticasone inhalers, twice daily as needed
Allergies
Family History
- No known family history of ocular disease
- Diabetes and hypertension
Social History
- Former smoker with a 40-pack-year tobacco use history
- No alcohol or drug use
- Competitive curler
- Works as a barber and owns his own business
Review of Systems
- Non-contributory, other than noted above
OCULAR EXAMINATION
Visual Acuity
- Right eye (OD): 20/20 at distance without correction
- Left eye (OS): 20/25 at distance without correction
Ocular Motility/Alignment
Intraocular Pressure
- OD: 12 mm Hg
- OS: 10 mm Hg
Pupils
- OD: 3 mm in dark, 2 mm in light, no relative afferent pupillary defect (RAPD)
- OS: 3 mm in dark, 2 mm in light, no RAPD
Confrontation visual fields
- OD: Full visual field
- OS: Small central relative scotoma, with central distortion on Amsler grid testing
External
Slit lamp exam
- Lids/Lashes: Normal OU
- Conjunctiva/Sclera: Normal OU
- Cornea: Normal OU
- Anterior chamber: Deep and quiet without flare or cell OU
- Iris: Normal architecture OU
- Lens: Normal OU
Dilated fundus examination (DFE)
- Vitreous: Normal OU
- Disc: Normal OU
- Cup-to-disc ratio: Normal OU
- Macula:
- OD: Subtle juxtafoveal pigmentary changes without visible subretinal fluid, lipid, or heme
- OS: Subretinal fluid beneath the fovea with subtle adjacent hypopigmentary changes superiorly; no visible lipid or heme
- Vessels: Normal OU
- Periphery: Normal OU
ANCILLARY TESTING
Fundus photography obtained at presentation is shown in Figure 1.
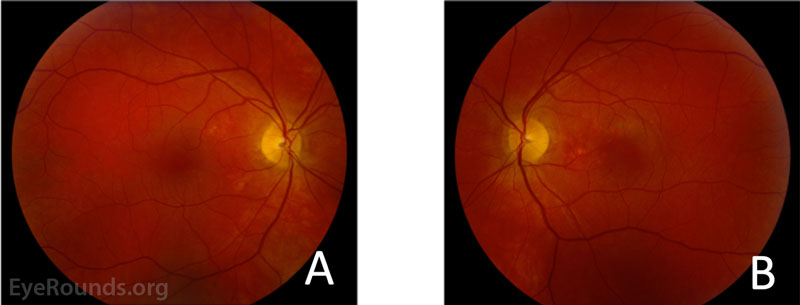
Figure 1: Fundus photography of the right (A) and left (B) eyes. The right eye demonstrated subtle hypopigmentary changes most apparent in the nasal macula, but no visible subretinal fluid, lipid, or heme. The left eye demonstrated subfoveal fluid with hyper- and hypopigmentary changes most prominent nasal and superior to the fovea.
Optical coherence tomography (OCT) obtained at presentation is shown in Figure 2.
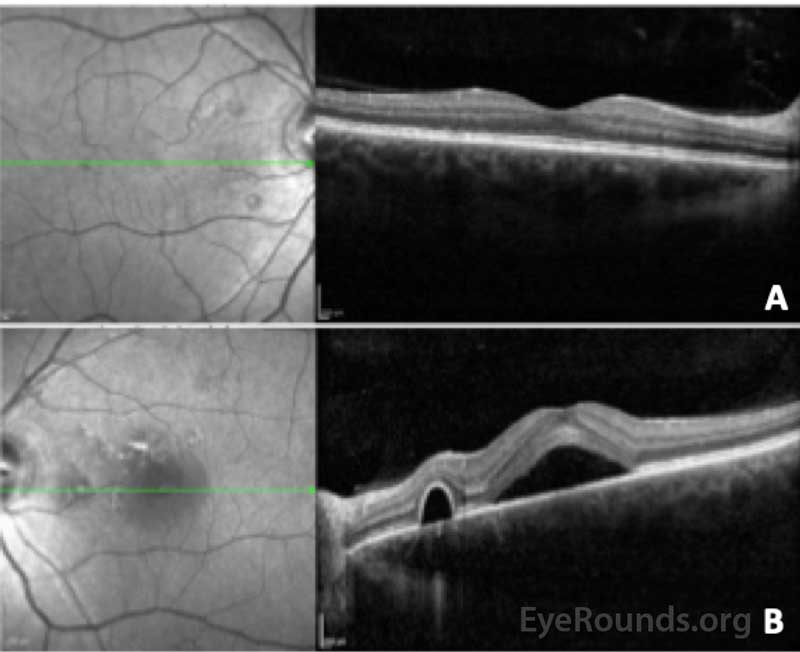
Figure 2: Optical coherence tomography of the right (A) and left (B) eye. In the right eye, the central macular thickness was 274 microns and there were several small serous pigment epithelial detachments nasal to the fovea without fluid. In the left eye, the central macular thickness 527 microns and there was subretinal fluid beneath the fovea with adjacent serous pigment epithelial detachments. The choroid was noted to be qualitatively thick in both eyes.
DIFFERENTIAL DIAGNOSIS
DIAGNOSIS
- Central Serous Chorioretinopathy (CSCR)
CLINICAL COURSE
Based on recent exogenous steroid use, macular pigmentary changes, subretinal fluid, serous pigment epithelial detachments, and a thick choroid in both eyes, the patient was diagnosed with central serous chorioretinopathy (CSCR). The decision was made to observe the subretinal fluid in the patient's left eye. He was encouraged to refrain from exogenous corticosteroid use as able and was counseled on the importance of stress reduction.
At his 6-week follow up visit, he reported improved vision in the left eye, with nearly resolved distortion. Visual acuity was 20/20 OU, and there was substantial reduction in the subretinal fluid in the left eye, as shown in Figure 3.
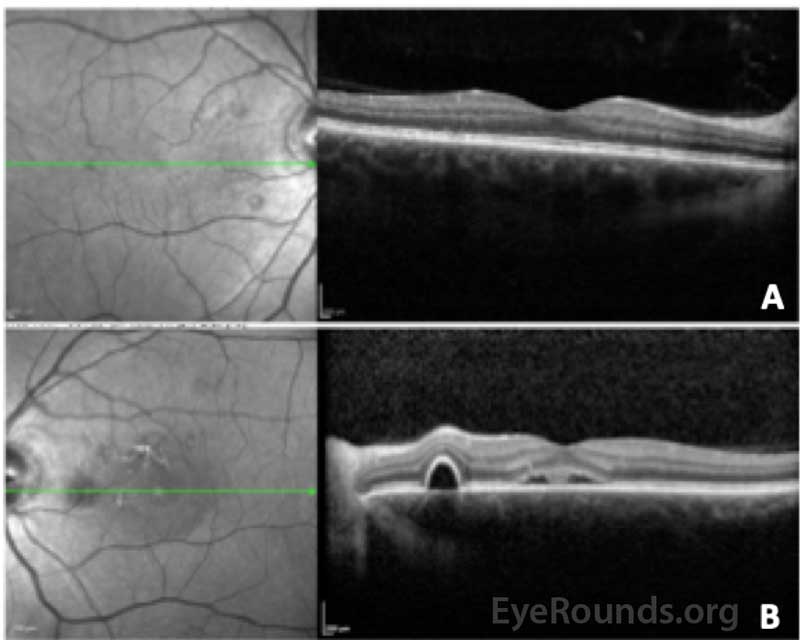
Figure 3: Optical coherence tomography of the right (A) and left (B) eyes at the 6-week follow up visit. The central macular thickness in the right eye was 275 microns (previously 274 microns) and there were several small serous pigment epithelial detachments nasal to the fovea without fluid. The central macular thickness in the left eye was 302 microns (previously 527 microns) and there was improved subfoveal fluid as well as smaller serous pigment epithelial detachments compared to previous.
DISCUSSION
Etiology/Pathophysiology
CSCR is a relatively common, idiopathic retinopathy that causes fluctuating vision loss, often affecting central vision. The incidence is around 10 in 10,000 individuals and is six times more likely to occur in men compared to women [1]. The age of onset is around age 40 and the mean age of affected men is lower compared to affected women [1]. Risk factors include endogenous hypercortisolism (Cushing's syndrome and pregnancy) and exogenous steroid use, including intravenous, oral, intraarticular, and intranasal administration [2 ,3]. Type A personality and various psychosocial stressors are also associated with CSCR [4]. Less common associations include collagen vascular diseases, systemic lupus erythematosus, sarcoidosis, obstructive sleep apnea, and the use of phosphodiesterase inhibitors [5-9].
The pathogenesis of serous fluid beneath the neurosensory retina in CSCR is not well established. One theory involves dysregulation of the retinal pigment epithelium (RPE), which normally creates a barrier between the neurosensory retina and the choroid, and actively transports fluid to dehydrate the subretinal space [10]. Another theory involves increased hyperpermeability of the choroidal circulation due to changes in local hydrostatic pressure, oncotic pressure, and vascular autoregulation. Helicobacter pylori infection has also been associated with CSCR, which may imply an inflammatory component present in this disorder [11].
Symptoms/Signs
Blurry central vision, scotomas, and metamorphopsia are common presenting symptoms. Other symptoms include dyschromatopsia, reduced contrast sensitivity, and micropsia [12]. Amsler grid testing is a useful tool to evaluate the size of the central scotomas. Fundus exam reveals circular areas of elevation in the macula that correspond to serous retinal detachments. Pigment epithelial detachments (PEDs) are commonly found [13]. Resolution of the macular serous fluid can be followed by atrophy of the RPE [14]. Choroidal neovascular membranes (CNVM) can occur as a complication of RPE atrophy in CSCR.
Optical coherence tomography (OCT) of the macula reveals detachment of the neurosensory retina, which may be associated with PEDs. The choroid is typically thick ("pachychoroid"), with dilated choroidal vessels [15 ,16]. Fundus fluorescein angiography (FFA) typically shows an expansile dot or "smokestack" leakage pattern of dye and can also be helpful to detect abnormal leakage within a CNVM. OCT-angiography (OCT-A) may also be useful to visualize abnormal vascular networks consistent with CNVM. In some cases, urinary free cortisol may be elevated, which can help establish the diagnosis in cases that are not straight forward [17].
Treatment/Prognosis
Most cases of acute or mild CSCR resolve spontaneously without treatment within several months [18]. Risk factor modification, such as cessation of exogenous steroid use, is important. Chronic or severe CSCR may be treated with oral epleronone or spironolactone [19 ,20]. These medications are potassium-sparing diuretics and are thought to be helpful in treating CSCR due to their mineralocorticoid receptor antagonism. Common side effects of these drugs include gyneocomastia and hyperkalemia, which should be monitored for regularly. Photodynamic therapy (PDT) to sources of leakage identified on FFA or indocyanine green angiography (ICG) is a useful treatment option in recalcitrant cases, or those where central vision loss is disabling and faster recovery is desired [21-23].
The visual prognosis is excellent for most cases of CSCR, but recurrences are common [24]. Return of subretinal fluid most frequently occurs during the first year after initial diagnosis but can also occur many years later [24]. Severe or chronic CSCR has a more guarded prognosis and can lead to permanent vision loss [25]. Long-term sequelae resulting in vision loss include thinning of the neurosensory retina, geographic atrophy, and CNVM [26].
Epidemiology and Etiology
- Most common in men around age 40
- Risk factors include exogenous or endogenous corticosteroids, stress, type A personality disorders, and others
- Pathophysiology involves hyperpermeability of the choroidal vasculature
|
Signs
- Circular areas of elevated retina within the macula, pigment epithelial detachments, small areas of retinal pigment epithelial atrophy, and in severe cases choroidal neovascular membranes
- OCT reveals subretinal fluid, pigment epithelial detachments, and thickened areas of choroidal vessels
- Fundus fluorescein angiography reveals "smokestack" leakage pattern
- OCT-A may show areas of choroidal neovascular membranes
- Urinary cortisol may be elevated
|
Symptoms
- Subacute painless vision loss characterized as blurry vision, central scotoma, metamorphopsias, decreased contrast sensitivity, and color discrimination
|
Management
- Risk factor modification and observation
- Anti-androgenic agents such as epleronone and spironolactone with potassium lab draws every 6 weeks to monitor for hyperkalemia
- Photodynamic therapy
|
References
- Kitzmann AS, Pulido JS, Diehl NN, Hodge DO, Burke JP. The incidence of central serous chorioretinopathy in Olmsted County, Minnesota, 1980-2002. Ophthalmology 2008;115(1):169-173. https://www.ncbi.nlm.nih.gov/pubmed/18166410
- Carvalho-Recchia CA, Yannuzzi LA, Negrao S, Spaide RF, Freund KB, Rodriguez-Coleman H, Lenharo M, Iida T. Corticosteroids and central serous chorioretinopathy. Ophthalmology 2002;109(10):1834-1837. https://www.ncbi.nlm.nih.gov/pubmed/12359603
- Quillen DA, Gass DM, Brod RD, Gardner TW, Blankenship GW, Gottlieb JL. Central serous chorioretinopathy in women. Ophthalmology 1996;103(1):72-79. https://www.ncbi.nlm.nih.gov/pubmed/8628563
- Yannuzzi LA. Type-A behavior and central serous chorioretinopathy. Retina 1987;7(2):111-131. https://www.ncbi.nlm.nih.gov/pubmed/3306853
- Cunningham ET, Jr., Alfred PR, Irvine AR. Central serous chorioretinopathy in patients with systemic lupus erythematosus. Ophthalmology 1996;103(12):2081-2090. https://www.ncbi.nlm.nih.gov/pubmed/9003342
- Fraunfelder FW, Fraunfelder FT. Central serous chorioretinopathy associated with sildenafil. Retina 2008;28(4):606-609. https://www.ncbi.nlm.nih.gov/pubmed/18398363
- Leveque TK, Yu L, Musch DC, Chervin RD, Zacks DN. Central serous chorioretinopathy and risk for obstructive sleep apnea. Sleep Breath 2007;11(4):253-257. https://www.ncbi.nlm.nih.gov/pubmed/17457629
- Sharma OP, Rao N, Roy M. Sarcoidosis and central serous retinopathy: a dangerous combination. Sarcoidosis Vasc Diffuse Lung Dis 1998;15(2):189-191. https://www.ncbi.nlm.nih.gov/pubmed/9789899
- Liew G, Quin G, Gillies M, Fraser-Bell S. Central serous chorioretinopathy: a review of epidemiology and pathophysiology. Clin Exp Ophthalmol 2013;41(2):201-214. https://www.ncbi.nlm.nih.gov/pubmed/22788735
- Negi A, Marmor MF. The resorption of subretinal fluid after diffuse damage to the retinal pigment epithelium. Invest Ophthalmol Vis Sci 1983;24(11):1475-1479. https://www.ncbi.nlm.nih.gov/pubmed/6642927
- Cotticelli L, Borrelli M, D'Alessio AC, Menzione M, Villani A, Piccolo G, Montella F, Iovene MR, Romano M. Central serous chorioretinopathy and Helicobacter pylori. Eur J Ophthalmol 2006;16(2):274-278. https://www.ncbi.nlm.nih.gov/pubmed/16703546
- Williams CM. Visual acuity and colour vision tests--a preliminary report. Br J Physiol Opt 1976;31(4):29-31. https://www.ncbi.nlm.nih.gov/pubmed/1053022
- Mitarai K, Gomi F, Tano Y. Three-dimensional optical coherence tomographic findings in central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol 2006;244(11):1415-1420. https://www.ncbi.nlm.nih.gov/pubmed/16596405
- Bujarborua D. Long-term follow-up of idiopathic central serous chorioretinopathy without laser. Acta Ophthalmol Scand 2001;79(4):417-421. https://www.ncbi.nlm.nih.gov/pubmed/11453866
- Cheung CMG, Lee WK, Koizumi H, Dansingani K, Lai TYY, Freund KB. Pachychoroid disease. Eye (Lond) 2019;33(1):14-33. https://www.ncbi.nlm.nih.gov/pubmed/29995841
- Gallego-Pinazo R, Dolz-Marco R, Gomez-Ulla F, Mrejen S, Freund KB. Pachychoroid diseases of the macula. Med Hypothesis Discov Innov Ophthalmol 2014;3(4):111-115. https://www.ncbi.nlm.nih.gov/pubmed/25756060
- Garg SP, Dada T, Talwar D, Biswas NR. Endogenous cortisol profile in patients with central serous chorioretinopathy. Br J Ophthalmol 1997;81(11):962-964. https://www.ncbi.nlm.nih.gov/pubmed/9505819
- Gilbert CM, Owens SL, Smith PD, Fine SL. Long-term follow-up of central serous chorioretinopathy. Br J Ophthalmol 1984;68(11):815-820. https://www.ncbi.nlm.nih.gov/pubmed/6541945
- Bousquet E, Beydoun T, Zhao M, Hassan L, Offret O, Behar-Cohen F. Mineralocorticoid receptor antagonism in the treatment of chronic central serous chorioretinopathy: a pilot study. Retina 2013;33(10):2096-2102. https://www.ncbi.nlm.nih.gov/pubmed/23719402
- Chin EK, Almeida DR, Roybal CN, Niles PI, Gehrs KM, Sohn EH, Boldt HC, Russell SR, Folk JC. Oral mineralocorticoid antagonists for recalcitrant central serous chorioretinopathy. Clin Ophthalmol 2015;9:1449-1456. https://www.ncbi.nlm.nih.gov/pubmed/26316684
- Yannuzzi LA, Slakter JS, Gross NE, Spaide RF, Costa D, Huang SJ, Klancnik JM, Jr., Aizman A. Indocyanine green angiography-guided photodynamic therapy for treatment of chronic central serous chorioretinopathy: a pilot study. Retina 2003;23(3):288-298. https://www.ncbi.nlm.nih.gov/pubmed/12824827
- Ober MD, Yannuzzi LA, Do DV, Spaide RF, Bressler NM, Jampol LM, Angelilli A, Eandi CM, Lyon AT. Photodynamic therapy for focal retinal pigment epithelial leaks secondary to central serous chorioretinopathy. Ophthalmology 2005;112(12):2088-2094. https://www.ncbi.nlm.nih.gov/pubmed/16325707
- Erikitola OC, Crosby-Nwaobi R, Lotery AJ, Sivaprasad S. Photodynamic therapy for central serous chorioretinopathy. Eye (Lond) 2014;28(8):944-957. https://www.ncbi.nlm.nih.gov/pubmed/24946843
- Ficker L, Vafidis G, While A, Leaver P. Long-term follow-up of a prospective trial of argon laser photocoagulation in the treatment of central serous retinopathy. Br J Ophthalmol 1988;72(11):829-834. https://www.ncbi.nlm.nih.gov/pubmed/3061449
- Spaide RF, Campeas L, Haas A, Yannuzzi LA, Fisher YL, Guyer DR, Slakter JS, Sorenson JA, Orlock DA. Central serous chorioretinopathy in younger and older adults. Ophthalmology 1996;103(12):2070-2079; discussion 2079-2080. https://www.ncbi.nlm.nih.gov/pubmed/9003341
- Yannuzzi LA, Shakin JL, Fisher YL, Altomonte MA. Peripheral retinal detachments and retinal pigment epithelial atrophic tracts secondary to central serous pigment epitheliopathy. Ophthalmology 1984;91(12):1554-1572. https://www.ncbi.nlm.nih.gov/pubmed/6084221
Suggested citation format:
Hendricks TM, Quist TS, Han IC. Central Serous Chorioretinopathy. EyeRounds.org. Posted January 28, 2020; Available from https://EyeRounds.org/cases/291-central-serous-chorioretinopathy.htm
last updated: 1/28/2020
Image Permissions:

Ophthalmic Atlas Images by EyeRounds.org, The University of Iowa are licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License.
