CASE #1
INITIAL PRESENTATION
Chief Complaint: Floaters and decreased vision in both eyes
History of Present Illness:
An 84-year-old man with Alzheimer’s dementia presented with floaters and decreased vision in both eyes (OU). His past ocular history was significant for a remote history of acute retinal necrosis (ARN) in his right eye (OD) over thirty years prior to presentation. This was thought to be related to varicella-zoster virus (VZV), as it occurred in the context of herpes zoster ophthalmicus (distribution of cranial nerve V1) on the right side. His previous episode resolved with intravenous and oral acyclovir as well as oral prednisone. Due to the degree of necrosis and high risk for detachment, he underwent pars plana vitrectomy, scleral buckle, and laser in the right eye, and he subsequently developed multiple additional retinal breaks which were treated with cryopexy and laser photocoagulation.
He remained stable until his present episode, when he noticed floaters and decreased vision in his right eye. He was seen at an outside eye clinic and found to have keratic precipitates (KP) and anterior chamber cell OD, consistent with acute anterior uveitis. He was started on topical prednisolone acetate 1% drops every 2-4 hours and cyclopentolate 1% three times daily. The anterior chamber cell persisted at one week, and he was started on oral valacyclovir 1g three times daily for possible viral-related iritis. However, the patient self-discontinued valacyclovir after a few days due to the bad taste.
On routine follow up a week later, vision was reportedly stable in the right eye, but vision in the left eye (OS) had decreased from a baseline of 20/20 to hand motion only, so he was referred to the University of Iowa for further evaluation.
Past Ocular History:
OD
OS
Past Medical History
Medications
Allergies: No known drug allergies
Family History: Non-contributory. However, his wife had shingles 1-2 months prior to his presentation. He suffered no symptoms at the time of her infection.
Social History: Former pipe smoker
Review of Systems: Negative except for what is detailed in the history of present illness.
OCULAR EXAMINATION
IMAGING
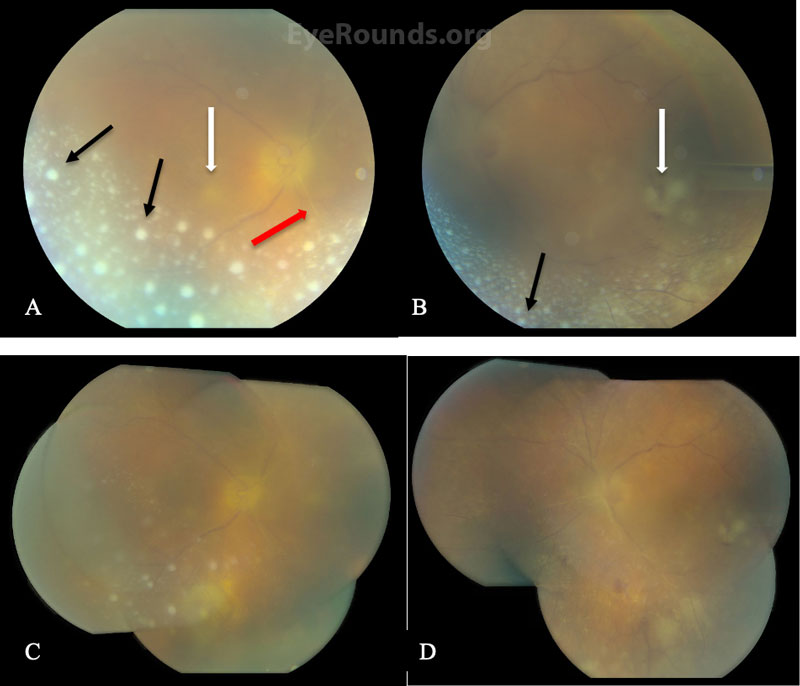
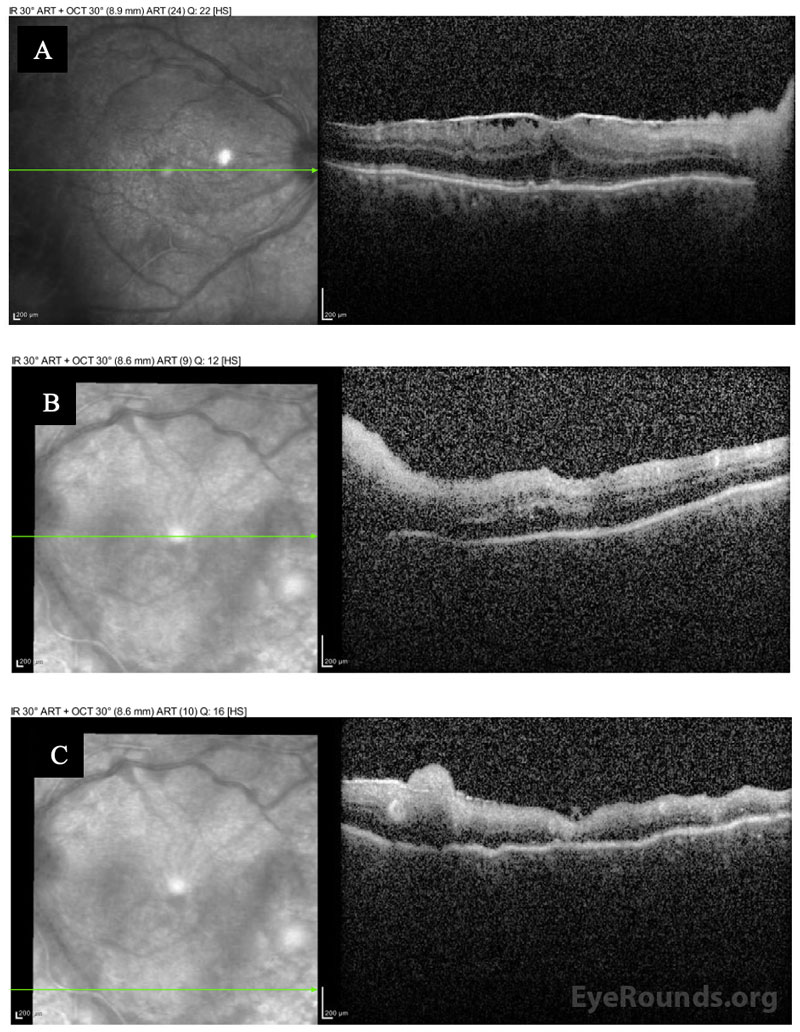
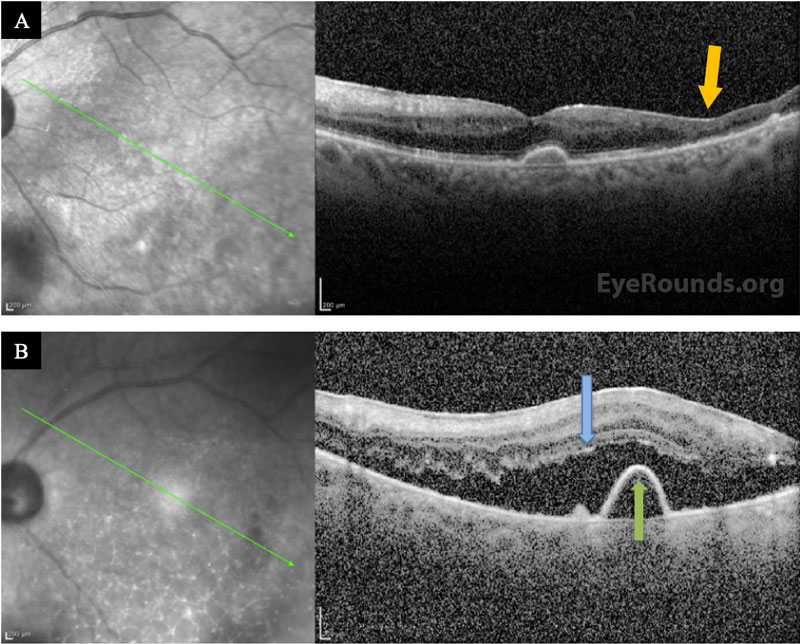
Differential Diagnosis:
CLINICAL COURSE
The presence of bilateral panuveitis, homogeneous areas of retinal whitening, arteriolar sclerosis, and disc edema were concerning for ARN, particularly given the patient’s history of VZV-associated ARN years prior. Given the disc and macular involvement, the patient underwent a vitreous tap and intravitreal injection of foscarnet 2.4mg/0.1ml in both eyes and, due to his prior inability to tolerate oral valacyclovir, was admitted as an inpatient for IV acyclovir 15mg/kg/day. He was also continued on topical steroids (prednisolone acetate 1% drops every 4 hours) and cycloplegia (atropine 1% drops twice daily) in both eyes.
Given the atypical appearance, a limited workup for other potential causes of panuveitis or retinitis was performed. Laboratory results were normal or negative for angiotensin-converting enzyme (ACE), syphilis IgG, human immunodeficiency virus (HIV), tuberculosis (QuantiFERON Gold), and toxoplasma (IgG or IgM). Polymerase chain reaction (PCR) testing on the vitreous tap was positive for VZV but negative for HSV and CMV.
The patient was followed daily and started on oral prednisone 40 mg within 48 hours after initial improvement. He underwent a second intravitreal injection of foscarnet in both eyes three days after the initial treatment. Within a week, he could see colors again, objects were clearer, and he felt no eye pain. He was discharged to a skilled nursing facility and completed a total 3-week course of IV acyclovir after having resolution of retinal whitening. He was then transitioned to a maintenance dose of oral valacyclovir (1g daily), which he tolerated without side effects. His visual acuity recovered to 20/125-1 OD and 20/80-1 OS, but he had episodes of vivid visual hallucinations in the context of his severe vision loss, consistent with Charles Bonnet Syndrome. Seven months after presentation, his OCT showed severe retinal atrophy in areas but no detachment (Figure 3A).
Unfortunately, within the year after this episode, the patient’s mental status declined due to worsening Alzheimer’s dementia. Over a year after initial presentation, the patient’s daughter reported that her father’s vision had declined over a period of 3 weeks. He was missing objects on his left side and could not easily identify faces. On exam, his visual acuity was 20/400 OD and 20/500 OS. There were no signs of intraocular inflammation or active retinitis in either eye, but he was found to have a macula-involving rhegmatogenous retinal detachment in the left eye (Figure 3B) for which he underwent repair with vitrectomy, scleral buckle, and placement of silicone oil in the left eye. Although the retina remained attached under oil, the patient continued to decline cognitively and further subjective assessments of his visual function were difficult.
DIAGNOSIS: Acute Retinal Necrosis
DISCUSSION
Etiology/Epidemiology
ARN is a viral panuveitis syndrome characterized by vitritis and vasculitis of the retinal and choroidal vessels with resultant necrotizing retinitis that preferentially affects the peripheral retina. It was first described in 1971 by Urayama in Japan, who documented six cases of previously immunocompetent patients with panuveitis and widespread retinal necrosis with vasculitis who subsequently went on to develop retinal detachment [1]. Since then, histologic studies have linked this vasocclusive, necrotizing retinitis to viral etiologies [2,3]. VZV has been found to be the most common etiology of ARN in immunocompetent patients, followed by HSV. Epstein-Barr virus (EBV) and CMV are rarer causes, with CMV being more common among immunocompromised individuals [4–6].
Two nationwide epidemiologic studies in the United Kingdom have estimated the minimum incidence to be between 0.5 and 0.63 cases per million per year [5,6] although this is likely an underestimation based on underreporting. Male gender was found to be a risk factor for severe visual loss from ARN [8], [9]. ARN can affect patients of any age, although the specific virus may be different: HSV-2 is a more common etiology in younger patients while VZV and HSV-1 are more common in older patients [1,2], [5]. Given the rising elderly population, the incidence of ARN is speculated to increase in the coming years [6,7].
Pathophysiology
Most cases of ARN are caused by the reactivation of a latent herpes virus, and it is theorized that dormant virus in the central nervous system spreads trans-axonally through the optic nerve to reach the tissues of the eye [1], [10-12]. However, in rare cases, ARN is caused by a primary infection, possibly reaching the eye via infected lymphocytes which are able to travel hematogenously and cross the blood-retina barrier [1].
The retinal necrosis seen in ARN likely occurs through two mechanisms: infection and ischemia. Intracellular viral replication directly induces necrosis and cytolysis of retinal cells. Additionally, the presence of viral antigens provokes an inflammatory immune-complex-mediated response resulting in lymphocyte infiltration into the retinal tissue and vasculature, causing a vasculitis that obliterates the retinal and choroidal vasculature. Resultant vascular occlusion creates ischemic necrosis within the retina, compounding the damage directly inflicted by the virus [1,13]. Like the retina, the optic nerve can be damaged by direct viral infection, ischemic necrosis secondary to perineural vasculitis, and loculated exudate within the optic nerve sheath which can cause compressive ischemia [1,4].
The vitreous also undergoes an inflammatory cellular reaction and is clouded by necrotic cells which have sloughed off the retina. In the anterior chamber, patients can have a fine or granulomatous inflammatory reaction that is generally less chronic and severe than the posterior segment reaction [1].
The inflammatory phase of ARN is self-limited, and without treatment, inflammation typically subsides within 6-12 weeks after the onset of symptoms, though the retina is left thin and atrophic with damaged underlying retinal pigment epithelium [1,13]. The vitreous becomes fibrous and opaque, developing contractile membranes and creating traction on the retina that is similar to proliferative vitreoretinopathy (PVR) [1], [13,14]. The combination of full-thickness retinal necrosis and traction can lead to retinal tears and ultimately detachment, usually within weeks to months after the initial infection [1].
Signs/Symptoms:
ARN is a clinical diagnosis based on clinical criteria created by the American Uveitis Society in 1994. Their criteria include peripheral retinal necrosis, progression of necrosis in the absence of treatment, occlusive vasculopathy, and anterior and/or posterior uveitis. Optic atrophy, scleritis, and pain may support the diagnosis but are not always present [15]. Other exam findings can include conjunctival injection, aqueous flare and cells, fine or mutton fat keratic precipitates, increased intraocular pressure, retinal arteriole sheathing, retinal hemorrhage, and optic disc edema [13,16].
Common early visual symptoms can include blurry vision, floaters, and decreased visual acuity. Patients may present with acute onset of ocular or periorbital pain, pain with eye movement, redness, photophobia, and foreign body sensation, although pain and redness can be absent [1], [13]. Long term complications include retinal atrophy, retinal detachment, pigmentary retinopathy, optic atrophy, cystoid macular edema, macular hole, epiretinal membrane formation, glaucoma, and cataract with posterior synechia [7,13], all of which can further add to visual decline.
Testing/Laboratory work-up:
PCR analysis of vitreous or aqueous fluid has a sensitivity of 95% and specificity of 97% in detecting viral etiology and has replaced viral cultures and antibody testing as the gold standard for diagnosis [1,4,7,12,17]. In fact, cultures can be negative even when electron microscopy demonstrates evidence of viral infection [1]. Retinal biopsy is rarely necessary but can be considered in cases when the clinical picture is atypical and PCR and culture are not helpful [17]. Serum titers for VZV and HSV can also be drawn, although they are often not positive and may have limited diagnostic value [1].
Treatment should not be postponed to wait for a PCR result. However, before starting anti-viral therapy (e.g., acyclovir), obtaining a complete blood count, creatinine, blood urea nitrogen, and liver function tests is necessary to monitor for complications of treatment. It is also important to rule out tuberculosis with the QuantiFERON Gold or purified protein derivative skin test and a chest x-ray before starting corticosteroids. Fluorescent treponemal antibody absorption, rapid plasma reagin, erythrocyte sedimentation rate, toxoplasmosis titers, ACE levels, and HIV may also be obtained to rule out other etiologies [1,13,17].
Imaging:
Fundus photography is useful in monitoring disease progression but may be limited by media opacity in the acute phases of inflammation [13]. Fluorescein angiography (FA) can demonstrate vascular leakage, and abrupt termination of filling within both arteries and veins consistent with occlusive vasculitis may be seen [1,13]. OCT may be used to monitor posterior regions of retinitis or evaluate for disc edema and long-term sequelae (e.g. cystoid macular edema and epiretinal membrane formation) [13]. Ocular echography is used to detect retinal detachment when the ocular exam is hindered by dense vitritis, and can also occasionally detect enlargement of the optic nerve sheath in cases of optic nerve edema [1]. Brain CT or MRI as well as lumbar puncture can be used in cases where viral encephalitis is suspected, or to evaluate other potential etiologies such as intraocular lymphoma or syphilis [13]. CT may demonstrate optic nerve sheath enlargement, and MRI may reveal lesions of the optic tract, chiasm, and lateral geniculate body [1].
Treatment/Management/Guidelines:
Currently, the preferred practice pattern for ARN is a 7-10 day course of intravenous acyclovir followed by an oral course of antivirals for 6-12 weeks, depending on treatment response [3,5], [13,18]. Because ARN is self-limited even without treatment, some argue that the main goal of antiviral therapy is to prevent involvement of the contralateral eye [13]. Contralateral eye involvement is seen in 13% of patients treated with antivirals, whereas up to 70% of untreated patients develop ARN in the second eye [3,7]. Due to the relative rarity of the disease and heterogeneity of manifestations, no randomized controlled trials comparing different treatment regimens have been conducted [1,5].
Emerging evidence has shown that the efficacy of oral antivirals is likely comparable to intravenous therapy. For this reason, oral antivirals alone have become an acceptable alternative to intravenous treatment [3,4, 13,18]. Oral antivirals, such as valacyclovir and famciclovir, have greater bioavailability and central nervous system (CNS) penetration than intravenous acyclovir [2–4], and they come with the added benefits of better affordability and convenience compared to IV acyclovir, which often requires inpatient hospitalization for treatment [3].
Intravitreal antiviral injections are often used in the treatment of severe ARN as an adjunct to systemic antivirals to quickly achieve high concentrations of antivirals in the retina and vitreous [19]. Although intravitreal injections of foscarnet and ganciclovir have proven efficacy, the indications for their use and dosing have not been established [20]. Certain institutions will treat with injections if the retinitis involves the optic nerve or macula or if retinal necrosis continues to progress after the administration of systemic antivirals [21].
Corticosteroids can mitigate the harmful side effects of the inflammatory response in ARN, particularly when the optic nerve is involved, but should only be given 24-48 hours after the administration of antivirals. If given before antivirals, corticosteroids may increase the risks of fulminant disease [7,13].
Hyperaggregation of platelets has been demonstrated in ARN and appears to contribute to the occlusive nature of the vasculitis [16]. Many studies have incorporated small doses of aspirin into treatment regimens in an effort to combat this effect, and it has been suggested that warfarin and heparin may prevent occlusion. However, there is minimal evidence that use of anticoagulants improves outcomes [1,7,13].
ARN carries a poor visual prognosis overall, depending on the degree of optic nerve or macular involvement, and due to the high risk of retinal detachment. Approximately 50% of cases have a visual acuity worse than 20/200 six months after onset [2,3]. Retinal detachment is estimated to affect up to 75% of patients [1,3], and prophylactic treatment with laser photocoagulation to barricade areas of retinitis or early pars plana vitrectomy with silicone oil tamponade and/or scleral buckle placement are sometimes used, but the effect of these preventive measures remains a matter of debate [1], [4]. In severe cases, successful prevention and treatment of retinal detachment do not dramatically improve visual outcomes, likely due to co-involvement of optic nerve injury [1,4,18].
EPIDEMIOLOGY OR ETIOLOGY
|
DIAGNOSIS
|
LABS/IMAGING
|
TREATMENT/MANAGEMENT
|
References
Wang C, Wilson CM, Han IC. Case #1 - Acute Retinal Necrosis. EyeRounds.org. January 12, 2022. Available from https://eyerounds.org/cases/315-acute-retinal-necrosis.htm
CASE #2
INITIAL PRESENTATION
Chief Complaint: One week of painful, progressive vision loss in the left eye.
History of Present Illness:
A 44-year-old otherwise healthy male presented for a red left eye with some decreased vision. Patient stated he woke up a week prior to presentation at UIHC with a tearing and red left eye without purulent discharge. The vision was cloudy and there was initially no eye pain. He was evaluated by a local provider and was diagnosed with conjunctivitis. He was given topical gentamycin drops but noted no visual improvement after use of the drops. Four days after the initial presentation, he noticed his vision in the left eye acutely worsened and he began to note some eye pain. The morning prior to presentation in our clinic he was evaluated by a local ophthalmologist who referred him to the University of Iowa with concern for viral retinitis. There was no associated flashes of light or floaters. He denied any fevers, chills, skin changes and systemic symptoms including headache or neck pain. He had no history of immunocompromise.
Past Ocular History:
Past Medical History: None
Medications: None
Allergies:
Family History: Non-contributory.
Social History: Chews tobacco with occasional alcohol use.
Review of Systems: Non-contributory
OCULAR EXAMINATION
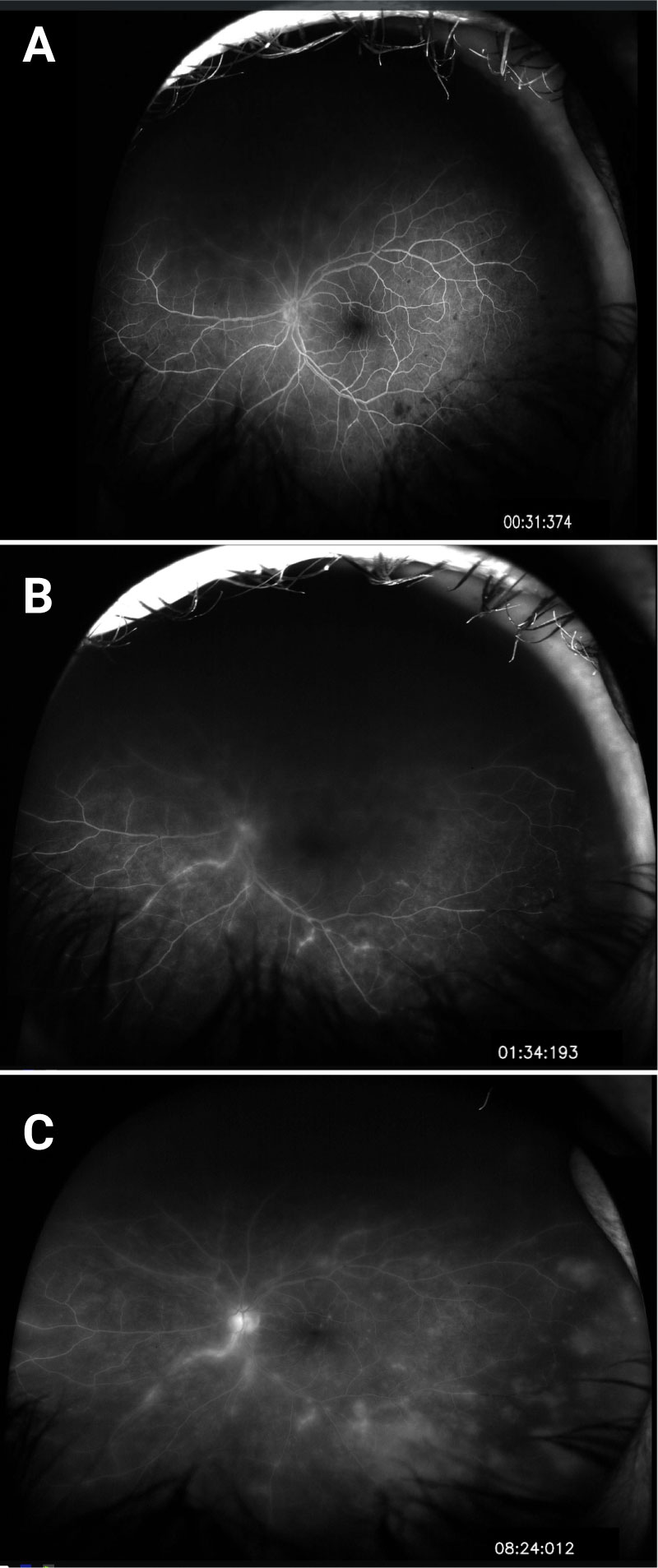
Differential Diagnosis:
CLINICAL COURSE
The patient initially presented to his local ophthalmologist but given the concern for acute retinal necrosis was sent to the University of Iowa for emergency intravitreal anti-virals, anterior chamber, and vitreous sampling for PCR.
Upon presentation to the University he was noted to have granulomatous inflammation and panuveitis in the left eye with retinal lesions suspicious for a viral etiology. For this reason, he was given intravitreal Foscarnet while serum and anterior chamber fluid samples were sent for PCR and culture analysis. Two days later, his anterior chamber fluid was positive for varicella zoster virus (VZV). He also had a positive serum titer for toxoplasmosis. It was determined that this clinical presentation was most consistent with acute retinal necrosis secondary to VZV infection, so the patient was started on prednisolone and atropine drops and oral Valacyclovir with close follow up.
He also received three more intravitreal Foscarnet injections over the next 10 days with noted improvement of the necrotic retinal lesions. Bactrim was also started empirically in the unlikely event that he had an active toxoplasmosis retinal infection. At one month after his initial appointment he was felt to be stable with 20/40 vision on maintenance prednisolone and oral anti-viral therapy. Unfortunately, when he returned for his two-month follow-up visit he had counting fingers vision in the left eye and reported a one-day history of acute inferior vision loss. He was found to have a superior macula off retinal detachment with grade C proliferative vitreoretinopathy (PVR). At that visit he reported only partial compliance with his topical and oral medications. He underwent retinal detachment repair by pars plana vitrectomy, endolaser, inferior retinotomy and placement of silicone oil tamponade. However, he was found to have re-detachment at his 3-week post-operative visit. He was then taken for re-repair which was successful, and the silicone oil was removed nine months later. Fortunately, the retina has remained attached and vision has since remained stable for the last five years with vision no better than 20/150 on consistent preventative maintenance therapy with oral acyclovir 1 gram daily. Chronic CME has been present and had minimal response to topical steroids, so they were not continued. He was referred to immunology for evaluation of subclinical immunodeficiency that might predispose him to such infections but he declined such testing so Valtrex 1 gm/day was continued empirically.
DIAGNOSIS: Acute Retinal Necrosis (secondary to varicella zoster infection)
DISCUSSION
Acute retinal necrosis (ARN) is a relatively rare inflammatory condition that was first described by Urayama et al in 1971 as a severe panuveitis with peripheral retinal necrosis classically associated with herpetic infections[1]. The hallmark characteristics include a severe retinal vasculitis accompanied by a full thickness necrosis to include the choroid and vitreous inflammation. Without treatment, this aggressive inflammation can progress to include retinal detachments and likely significant vision loss[2]. The patient in this case report represents a classic presentation of ARN secondary to herpes zoster in a young presumably immunocompetent man.
Etiology/Epidemiology
ARN syndrome is classically associated with an infection from the human herpesvirus family. Histopathologic evidence of this viral infection was first documented in 1982 which showed viral particles from the human herpes family within the retina, retinal pigment epithelium and vascular endothelial cells. This was further confirmed via positive serum titers for viral antigens and culture from ocular fluid specimens[3]. A majority of the literature for ARN supports this condition is most frequently caused by varicella zoster virus (VZV) but has also been reported secondary to herpes simplex virus (HSV) 1 and 2. Rare cases have also been associated with Epstein bar virus (EBV) and cytomegalovirus (CMV) infection[4].
Given this is a relatively rare condition, there is a paucity of literature about its epidemiologic state. Studies from the United Kingdom report an annual incidence of 0.63 cases per million per year[5, 6]. ARN can present as either unilateral or bilateral disease and has no known sex predilection [5]. Age of presentation can be variable, and both include children and adults. Historically, it is taught that ARN is a disease of an otherwise healthy individual however, more recent studies may elucidate evidence of subclinical immunocompromise as well as specific genetic risk factors involved in the immune response, that might make certain individuals at a higher risk for the disease.
Pathophysiology
Infection with the virus and the ensuing necrosis that follows is likely a combination of multiple factors. Acutely, the virus incites an inflammatory event causing damage to retinal cells and arterioles. It is the serve arteriolar inflammation that causes vaso-occlusive events ultimately leading to rapid necrosis of the retina and choroid. Histopathology of enucleated eyes with known ARN syndrome exhibit a full thickness inflammatory infiltration with necrotizing retinitis, granulomatous choroiditis, ischemic optic neuritis and atrophy, and perivascular invasion of the retinal arterioles [7]. Eosinophilic intranuclear inclusions within cells of all layers of the retina and retinal pigment epithelium were the first evidence of a viral etiology and more recently, VZV DNA and antigens have been identified in lymphoid cells within choroidal infiltrates [3,7]. Deposits of immune complexes containing these same viral antigens have also been demonstrated in retinal vessel walls which may contribute to the severe and occlusive vasculitis [3].
Although the classic teaching is that ARN syndrome is a disease with no identifiable predisposing risk factors, emerging evidence may suggest that there are some specific human leukocytic antigen (HLA) genotypes that are more disease “susceptible”. Various studies have found an increased association with disease between HLA-DQw7 and phenotype Bw62 and DR4 in Caucasians patients and HLA-Aw33, B44, and DRw6 in Japanese patients[8, 9].
It is important to distinguish ARN from other herpes related retinopathies, like progressive outer retinal necrosis syndrome or PORN which tends to affect those who are severely immunocompromised as seen in HIV infected patients. Clinically, PORN presents with multifocal patchy white lesions in the posterior pole, rather than the peripheral retina, which rapidly progress to involve the entire retina [10]. This can be unilateral or bilateral similar to ARN, but usually lacks the anterior chamber and vitreous inflammation, likely due to the patients weakened ability to amount an immune response. ARN may also have more prominent vaso-occlusive disease which can be more evident on fluorescein angiography[11]. Given the these two entities can manifest from the same viral etiology it has been surmised that these clinical descriptions may represent a spectrum of herpetic disease in patients with varying fundoscopic manifestations based on the amount of immune dysfunction[12]. However, more recent studies have highlighted cases with a clinical presentation of ARN in patients with HIV infection or some other impairment of cellular immunity, which may be subclinical [12]. Moreover, ARN and PORN have been documented in fellow eyes of a single immunocompromised patient which may further suggest that these two diseases represent a spectrum of herpetic related necrotizing retinopathies with varying manifestations depending on the degree of immunocompromise [13].
Signs/Symptoms:
ARN typically presents without any preceding illness in an otherwise healthy patient. The first symptoms may include pain and eye redness with some mildly decreased vision and photophobia coinciding with the acute anterior chamber reaction [2]. Within a few days of the acute inflammation there is a rapid and severe decrease in the visual acuity secondary to posterior segment involvement including marked vitreous inflammation, occlusive retinal arteritis, and whitening, hemorrhage, and necrosis of the retina and choroid. These necrotic patches often first present in the peripheral fundus but can become confluent and spread into the posterior pole [10]. Large retinal holes can develop in the necrotic retina followed by vitreous organization, and retinal detachment [14]. The detachment generally occurs 6–12 weeks after the onset of the disease and given the persistent vitreous inflammation and possible proliferative vitreoretinopathy can have both tractional and rhegmatogenous components. There is also a prominent and aggressive vasculitis, primarily affecting the arteries causing narrowing and sheathing [2]. Retinal phlebitis is a less prominent feature which may present as scattered retinal hemorrhages. The overall prognosis for visual acuity is generally poor, with only 30% of affected eyes achieving an acuity of better than 20/200 [10, 14].
Bilateral disease or BARN is rare and often does not present in the contralateral eye for several weeks or months. This was more frequent prior to the routine use of anti-viral therapy, like acyclovir, and more often indicates severe immune dysfunction or even iatrogenic immunosuppression like corticosteroids[15].
Diagnosis/Testing/Laboratory work-up:
Diagnostic criteria for ARN syndrome was first published in 1994 by the American uveitis society[16]. Although it has been associated with specific viral infections it remains a predominantly clinical diagnosis. The diagnostic criteria include specific clinical findings and noted progression with required and supportive elements as depicted in Table 1[16].
|
Required Clinical Criteria |
|
|
|
|
|
Supportive Clinical Features |
|
|
|
Given that ARN syndrome is defined clinically there is no single laboratory test to ensure diagnostic certainty. When clinical suspicion is high several methods of testing are available to aid in the diagnosis including intraocular fluid (vitreous or aqueous) antibody testing, viral culture, diagnostic vitrectomy, retinal biopsy and immunohistochemical staining [17]. Unfortunately, these methods can ensue some risks with low specificity and sensitivity making these tests sometimes of marginal net value. However, recent data suggests that polymerase chain reaction (PCR) analysis of vitreous and aqueous samples have a high specificity and may prove to be an added benefit in both the diagnosis and treatment of disease [17].
Imaging:
Although there is no one modality that can confirm the diagnosis of ARN several imaging studies exist that can assist to elucidate the cause and extent of the inflammation. Fluorescein angiogram may show early choroidal blockage due to decreased perfusion and abrupt “cut off” of retinal artery perfusion secondary to acute vaso-obstruction [2]. Later phases may also show leakage at the optic disc secondary to optic nerve edema and leakage of perifoveal capillaries due to macular edema [2]. Ultrasonography may also be useful to aid in diagnosis in the setting of significant vitreous inflammation and poor visualization to the posterior segment especially during active phases where it can detect uveal thickening, enlargement of the optic nerve sheath and retinal detachment [2,10]. Neuroimaging such as computed tomography way also be helpful as it may reveal optic nerve sheath enhancement and can help to rule out associated encephalitis and further viral extension into the central nervous system [2,10]. More recently, studies have shown that optical coherence tomography may be useful, when possible, showing hyperreflectivity in the inner retina, disorganization of the retinal layers, and subretinal fluid in the early phases, corresponding to the white-yellow necrotic lesions. Later, these areas will correspond to a dense hyperreflective band, likely secondary to retinal atrophy and scar formation [18].
Treatment/Management/Guidelines:
Given the most common etiology for ARN is herpetic, anti-viral oral intravenous and/or intravitreal therapy remains the treatment of choice. Currently, there is no one single standard treatment strategy. Blumertanz et al were the first to describe regression of the ARN lesions with intravenous acyclovir [19]. In addition, since this time intravenous acyclovir has been documented to reduce the incidence of infection in the contralateral eye [20]. However, oral anti-viral therapy with valacyclovir has also shown adequate bioavailability and given its ease of administration may be considered first line therapy in the future. It is important to note that the bioavailability of orally administered valacyclovir is superior to that of orally administered acyclovir so if a patient is to be treated with anti-virals orally for ARN, valacyclovir should be used [17, 21]. To date, there have been no studies to directly compare the efficacy of oral versus intravenous anti-viral therapy for the treatment of ARN. At present, the most common systemic treatment regimen is 10 mg/kg every 8 hours or 1500 mg/m2 per day for 7 to 10 days followed by an oral antiviral [17]. Intravitreal anti-viral treatment with Ganciclovir or Foscarnet are also utilized as adjuvant therapy which may provide the most direct medical therapy with the least amount of systemic side effects [17, 22, 23]. Intravitreal therapy may also be the medication of choice in patients with “acyclovir-resistant” ARN, although this is exceedingly rare and suggest an alternate diagnosis [24]. This most often is administered very early in the treatment regimen as it can be given directly after vitreous sampling, during the “tap and inject” procedure. These intravitreal injections can also be repeated in 48-72 hours [10]. Early surgical intervention, such as vitrectomy or laser retinopexy, may have promise in preventing secondary retinal detachment but unfortunately, despite the varying treatment strategies, the overall visual prognosis for ARN remains poor [25]. Accordingly, limited data exists about the benefit of long term prophylaxis with oral anti-viral treatment[26].
EPIDEMIOLOGY OR ETIOLOGY
|
SIGNS
|
SYMPTOMS
|
TREATMENT/MANAGEMENT
|
References
Rodriguez SM, Warren AK, Gehrs KM. Case #2 - Acute Retinal Necrosis. EyeRounds.org. January 12, 2022. Available from https://eyerounds.org/cases/315-acute-retinal-necrosis.htm

Ophthalmic Atlas Images by EyeRounds.org, The University of Iowa are licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License.