Chief Complaint: 55-year-old female with hazy vision in both eyes
History of Present Illness: The patient's first episode of blurry vision occurred six months prior to her initial visit at the University of Iowa. She had presented to her optometrist with one week of blurred vision in the left eye associated with photopsias and increased floaters. She was diagnosed with a hemorrhagic vitreous detachment and was referred to a local ophthalmologist, who agreed with the diagnosis.
Two months later, the patient's vision had become blurred in both eyes. When she saw her ophthalmologist, her vision was 20/30 in each eye, and she had diffuse pigmented cells in the vitreous OU. Her vision continued to worsen over the next few months, to the point where she had to stop driving because of extreme glare symptoms. She was then referred to the University of Iowa to evaluate and manage her non-clearing vitreous floaters.
Past Ocular History: High myopia (approximately -10.00 D OU)
Medical History: Patient has a known history of hypertension, first-degree AV block with a right bundle branch block, and a recent orthopedic procedure for the left foot.
Medications: None
Allergies: Patient is sensitive to wheat and feathers
Family History: Both parents had hypertension. Her father had a history of a myocardial infarction and his mother had a history of a stroke. Her mother and aunt had thyroid disease. Mother and paternal grandfather had arthritis. Ocular family history included maternal uncles with glaucoma, mother and maternal uncles with macular degeneration. One aunt had a retinal detachment.
Social History: The patient is an engineer. She uses occasional alcohol and has never smoked. She lives with her dogs and cats, but denies bites or scratches from the pets. She has exposure to chickens, pheasants, and quail. The chickens were diagnosed with coccidiomycoses recently. No recent travel or sick contacts.
Physical Exam:
| Visual Acuity | Glare Testing | |
|---|---|---|
| OD | 20/40 | 20/70 |
| OS | 20/30 | 20/30 |
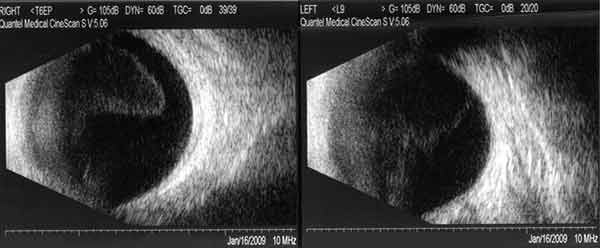 |
|---|
A laboratory workup was initiated for vitritis of unknown etiology. RPR, TPPA, CBC with differential, ACE level, and lysozyme were ordered. A PPD skin test was placed.
Over the next week all lab results returned as negative:
CBC: WBC 9.2K/mm3, Hb 14.5g/dL, Hct 43%, platelets 297K/mm3
RPR and TPPA: Non reactive
PPD: Non-reactive.
ACE level 17 U/L
Lysozyme 16 ug/mL
Course: The patient was started on Prednisone 40 mg QAM and returned 2 weeks later with improved vision (20/30+2 OD and 20/25+2 OS). The vitreous debris has partially cleared and small yellow spots were seen in the deep retina and choroid, suggestive of birdshot choroiditis. Macula OCT showed a small sub-foveal neurosensory detachment and epiretinal membrane (ERM) OD and a mild ERM OS.
HLA 29 antigen test was ordered and returned positive, further supporting the diagnosis of birdshot choroidopathy.
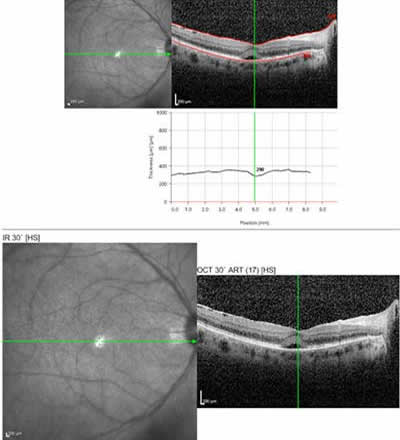 |
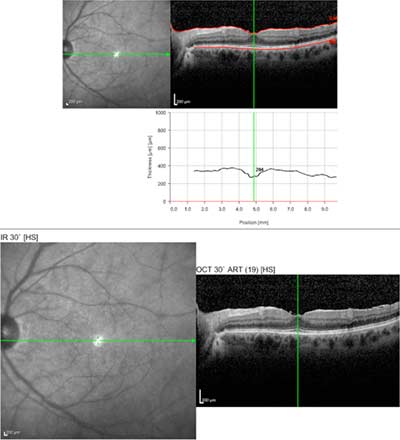 |
Follow up course: The patient continued to improve and the prednisone was tapered. However, on 15 mg Prednisone daily, the vitritis recurred and the vision worsened, The patient has been referred to a rheumatologist to start mycophenolate mofetil (CellCept) a, steroid-sparing agent.
Birdshot choroiditis (also known as vitiliginous chorioretinitis) was first described by Ryan and Maumenee in 1980 and by Gass in 1981. It is an uncommon disease that usually appears in healthy Caucasian women, in the third to sixth decades of life. To date, there is no published data on the prevalence of this disorder. However, a recent review of case reports and small retrospective cohort studies reported that 0.6-1.5% of patients who were referred to tertiary care centers for uveitis were diagnosed with birdshot chorioretinopathy, accounting for 6-7.9% of all posterior uveitides (Shah, et al). The literature reports that 52 to 58% of patients are women, with an average age of 48-53 years old.
Symptoms include blurred vision, floaters, and photopsias often followed by nyctalopia or problems with color vision. In the original paper by Ryan and Maumenee, the presenting symptoms were as follows:
Characteristic fundus lesions are described as multifocal, cream-colored, and ovoid and appear at the level of the RPE and choroid. They typically measure 50-1500 microns, and often extend radially from the optic nerve to cover the post-equatorial nasal retina. The lesions may appear and disappear during the course of the disease, making it a challenge to diagnose. Some have reported cases in which the lesions did not appear until years after the onset of vasculitis or optic disc edema. The macula is often spared, but a macular predominant type has been described. Ryan and Maumenee chose the name for this disorder because the chorioretinal lesions resembled birdshot scattering from a shotgun. The name "vitiliginous" is used occasionally because the depigmented choroidal lesions appear similar to the hypopigmented patches seen on the skin of patients with vitiligo.
The fundus appearance on fluorescein angiography varies depending on the chronicity of inflammation. Early in the disease, the lesions may appear silent or hypofluorescent in the early phase of the angiogram. Later in the disease, lesions show late hyperfluoresence indicting the presence of retinal and RPE atrophy, which generally follows the inflammation. ICG will show multiple hypofluorescent spots more numerous than that found on clinical examination and not necessarily corresponding with lesions seen on FA. Angiography may be more useful in depicting active retinal vasculitis, CME, and optic nerve head leakage. Progressive field loss and electroretinogram (ERG) abnormalities are commonly seen with extended follow up suggesting that retinal dysfunction may be more pervasive than indicated by the clinical exam. The ERG will show moderate to severely abnormal rod and cone function in both eyes of most patients.
Vision loss occurs primarily because of CME, optic neuropathy, or retinal and RPE atrophy, Neurosensory detachments in the macula and choroidal neovascular membranes can also result in vision loss. CME has been reported in up to 41%-50.5% of eyes with birdshot choroiditis. Without CME, however, patients may have good visual acuity.
North American and European individuals who are HLA-A29 positive, have a relative risk of 50-224 (88-98% correlation) compared to those without this haplotype for developing birdshot choroiditis. The relationship between HLA haplotypes and ocular inflammatory diseases is not specifically known but some theories include the following: HLA molecules bind to antigens or infectious agents that cross react with self-antigens, molecular mimicry exists between bacterial antigens and the HLA molecule itself, the T lymphocyte antigen receptor gene (which includes the HLA haplotype) makes one susceptible to inflammation, or having a specific HLA haplotype is associated with exaggerated innate immunity. HLA haplotyping should be used as a confirmatory test in the setting of convincing clinical signs, rather than as a diagnostic test because 7% of the general Caucasian population carries this haplotype, but most people clearly do not develop this chorioretinopathy.
There are very few histopathologic studies of eyes with birdshot choroiditis. In one case, a 49-year-old white male with birdshot lesions for 6 years who was never been treated with immunosuppressants had died of a myocardial infarction and underwent an autopsy (Gaudio, et al). Multiple foci of lymphocytes were found at various levels of the choroid, with some appearing around retinal blood vessels and in the prelaminar optic disc. They consisted predominantly of CD8+ T lymphocytes and less so by CD4+ T and B lymphocytes.
Birdshot choroiditis has been associated with retinal S-antigen. 50% of patients with birdshot show in vitro lymphocyte proliferation to purified retinal S-antigen. Because of its association with T-lymphocyte activation and similarity to known autoimmune inflammatory eye disease, it has been treated with steroids and steroid-sparing anti-inflammatory agents such as cyclosporine in particular. In a review by Shah et al, approximately 90% of patients who were treated with cyclosporine alone had stable (65.7%) or improved visual acuity (24.3%). No prospective controlled trials comparing cyclosporine to prednisone have been performed, but pooled retrospective studies and reports indicate that cyclosporine alone may be more effective than prednisone alone. Periocular steroids have been reportedly very helpful in managing CME and its recurrences. If oral steroids are used, they should be tapered slowly followed by maintenance with low dose steroids. If steroids are required for more than 3-4 months, then a second steroid-sparing agent should be added. Most patients need extended therapy with steroid-sparing agents.
The prognosis for these patients is relatively good with visual acuity stabilizing at around 20/30-20/40. 20% of patients have a self-limited form of the disease, but it is usually marked by multiple exacerbations and remissions. For those patients with disease duration of 30 months or more, however, 2/3 had a VA worse than 20/50 and 1/3 had VA worse than 20/200. The overall 5 year cumulative incidence of acuity of < or = 20/200 was 20% (Thorne, et al. from AAO Basic and Clinical Science Course Section 9).
EPIDEMIOLOGY
|
SIGNS
|
SYMPTOMS
|
TREATMENTDiagnostic work up includes: HLA-A29 antigen testing, FFA, ICG, +/- ERG. Treat CME and underlying inflammation with periocular or systemic immunosuppressants. Pericocular steroids are especially helping in managing CME and recurrences. Oral steroids should be tapered slowly followed by maintenance with low dose steroids. If steroids are required for more than 3-4 months, then a second steroid sparing agent should be added. Most patients need extended therapy.
|
Gass, D. Stereoscopic Atlas of Macular disease diagnosis and treatment, 3rd edition. J Donald M. Gass, 1987, Mosby, pp. 529-531.
Gaudio PA, Kaye DB, Crawford JB. Histopathology of birdshot retinochoroidopathy. Br J Ophthalmol. 2002;86(12):1439-41
Nussenblatt RB, Mittal KK, Ryan S, Green WR, Maumenee AE. Birdshot retinochoroidopathy associated with HLA-A29 antigen and immune responsiveness to retinal S-antigen. Am J Ophthalmol. 1982;94(2):147-58.
Nussenblatt, RB, Chapter 24 "Birdshot Retinochoroidopathy." Uveitis. Fundamentals and Clinical Practice. 3rd edition, Robert B Nussenblatt and Scott M Whitcup, 2004, Elsevier-Mosby, pp. 339-341.
Ryan SJ, Maumenee AE. Birdshot retinochoroidopathy. Am J Ophthalmol. 1980;89(1):31-45.
Shah K, et el. Birdshot Chorioretinopathy. Survey of Ophthalmology. 2005;50(6):519-541.
Thorne, et al. Intraocular Inflammation and Uveitis. Basic and Clinical Science Course Section 9, 2007. American Academy of Ophthalmology, Singapore, pp. 183-186.
Tsui JY, Graff J, Folk J. Birdshot Choroiditis: 55-year-old female with persistent vitreous floaters. EyeRounds.org. July 1, 2009; Available from: https://eyerounds.org/cases/96-birdshot-choroidopathy.htm

Ophthalmic Atlas Images by EyeRounds.org, The University of Iowa are licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License.