
Section 4-A: Introduction
The diagnosis of glaucoma has different implications for patients depending on the type of glaucoma diagnosed. Those with suspicious findings for glaucoma are called glaucoma suspects. These patients need to be watched carefully for the development of glaucoma. Crossing the line between being a suspect to actually being diagnosed with the disease depends on the development of optic nerve cupping with corresponding visual field loss. There are two categories of glaucoma when the diagnosis is made: open angle and angle-closure. Different risk factors (see Chapter 5) and treatments exist for each type.
Section 4-B: The Glaucoma Suspect
Patients are followed carefully for the development of glaucoma if they are deemed to be glaucoma suspect. There are various physical findings which may label someone as a glaucoma suspect. Physical findings which are suspect for glaucoma are large cup-to-disc ratios (see Chapter 1), asymmetric cupping between two eyes, and high intraocular pressures (IOP). Patients are suspicious for glaucoma based on optic nerve findings, such as large cups or asymmetric cupping –difference in cup size between the right and left eye (figure 4-1).
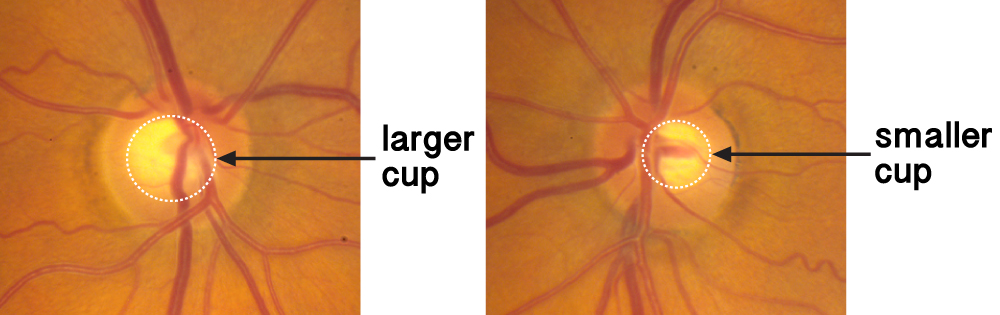
Figure 4-1. Asymmetry of cupping. The optic nerve cup at left is larger than the optic nerve cup at right.
Patients are also glaucoma suspect if they have elevated IOP or ocular hypertension. Normal IOP is between 10 to 21 mmHg. Those with ocular hypertension have IOPs which are greater than 21 mmHg. Accurate IOP measurement depends on the thickness of the cornea . Thin corneas may underestimate the IOP measurement since it is easier to press against the cornea when checking the IOP. Thus when the cornea is thin, the true IOP is higher than the measured IOP. Conversely, when the cornea is thick, the true IOP is lowered than the measured IOP. Corneal thickness measurements are made with a pachymeter. (figures 4-2 and 4-3) It is similar to the IOP check and performed with an eyedrop (topical) anesthesia. The IOP measurement is, therefore, combined with the corneal thickness measurement to ascertain true level of IOP.

Figure 4-2. A pachymeter tip used to measure corneal thickness.

Figure 4-3. A patient undergoes pachymetry to determine his corneal thickness.
Despite cupping or high IOPs, the visual field test is normal in glaucoma suspects. Progressing to glaucoma requires cupping with corresponding visual field loss. Those most at risk for the development to glaucoma are those with elevated IOPs, thin corneas (less than 555 microns), African descent, older age, enlarged cups, and a family history of glaucoma.
Optic nerve photographs are helpful in following glaucoma suspects. Comparison of optic nerves to previous photos allows eye doctors to determine if there is progression of optic nerve cupping. Changes in the optic nerve appearance over time indicate glaucomatous damage. A visual field test which is initially normal and begins to show signs of visual field loss indicates that glaucoma is developing. It is, therefore, important for glaucoma suspects to be carefully monitored with serial eye examinations over time. Since glaucoma often develops without symptoms, the first signs of glaucoma development may first be detected on examination by an eye doctor. Screening should be performed every 1-4 years after the age of 40 (see Chapter 2), depending on a patient’s risk factors. Those with more risk factors are examined more frequently than those without risks.
Section 4-C: Open-Angle Glaucoma
One major type of glaucoma is open-angle glaucoma. The difference between open-angle and angle closure glaucoma is based on examination. The term angle (short for irido-corneal angle) refers to the drainage angle of the eye, which is between the cornea and the iris. Those with open-angle glaucoma have a widely open drainage angle on examination. The angle is examined with a special lens called a gonioscopy lens (“gonio” means angle). (Figure 4-4)
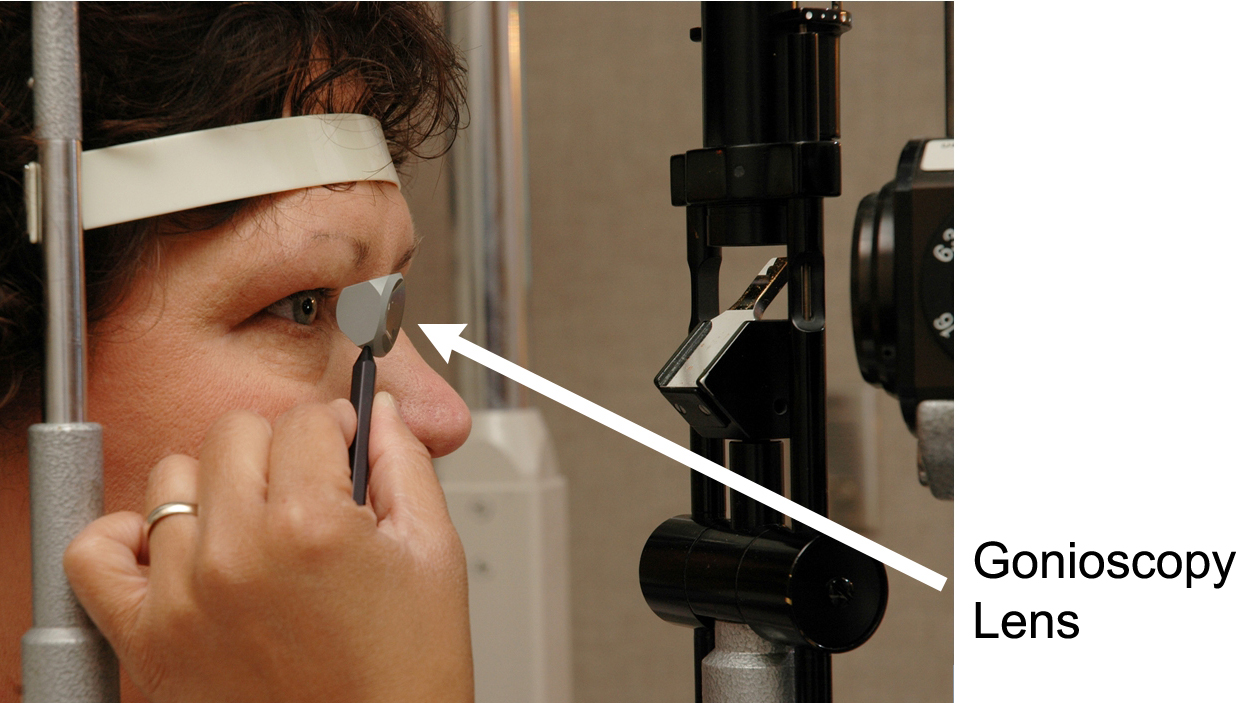
Figure 4-4. A patient undergoing gonioscopy
An open-angle with cupping of the optic nerve and glaucomatous visual field loss leads to the diagnosis of open-angle glaucoma. There are primary and secondary causes of open-angle glaucoma. If there is no identifiable factor causing the glaucoma (i.e., the cause of the glaucoma is unknown), this is referred to as primary open-angle glaucoma (POAG) or chronic open-angle glaucoma (COAG). If there is a known process leading to decreased fluid drainage through the angle, such as blood, inflammatory cells, or pigment, this is called secondary open-angle glaucoma. Glaucoma associated with pigment dispersion syndrome and pseudo-exfoliation syndrome are examples of secondary open-angle glaucoma.
POAG is the most common form of glaucoma in the U.S. POAG can be of two types. It can be associated with either elevated IOP or normal IOP. The former is referred to as POAG with elevated IOP or high pressure glaucoma. The latter is often referred to as normal tension glaucoma (NTG) or low tension glaucoma. NTG has the same characteristics as POAG except the IOP is in the normal range, that is, less than 21 mmHg. One theory regarding the mechanism of injury in NTG is insufficient blood flow leading to optic nerve damage. The POAG and NTG may represent a spectrum of disease all leading to visual loss.
Although NTG patients have IOPs less than 21 mmHg, it has been shown that lowering the IOP to low-normal or even sub-normal range halts or slows the progression of glaucomatous damage. Aside from the IOP, the examination findings are very similar between POAG and NTG. A few differences have been observed. More optic nerve hemorrhages (bleeding spots) are found in NTG than in patients with POAG. The visual field test may also show more central (as opposed to peripheral) loss in NTG. These differences are not absolute and many of the characteristics between the two forms overlap.
The treatment goal of open-angle glaucoma is to lower IOP. This is more challenging in NTG, but lowering IOP has been shown to be effective even in NTG as well as POAG. Treatment modalities include topical medications (glaucoma eye drops), laser to the drainage area or trabecular meshwork, and filtering surgery. Medical and surgical treatment will be covered in more depth in Chapters 7 and 8.
Section 4-D: Angle Closure Glaucoma
Angle closure glaucoma (also called closed-angle glaucoma) may present very differently from open-angle glaucoma. In contrast to open-angle glaucoma which is mostly asymptomatic, (acute) angle closure glaucoma may present suddenly with pain, nausea, and decreased vision. As its name implies, the drainage angle is closed when examined with a gonioscopy lens. (figure 4-5)
.jpg)
Figure 4-5. The goniolens on the eye examining the drainage angle
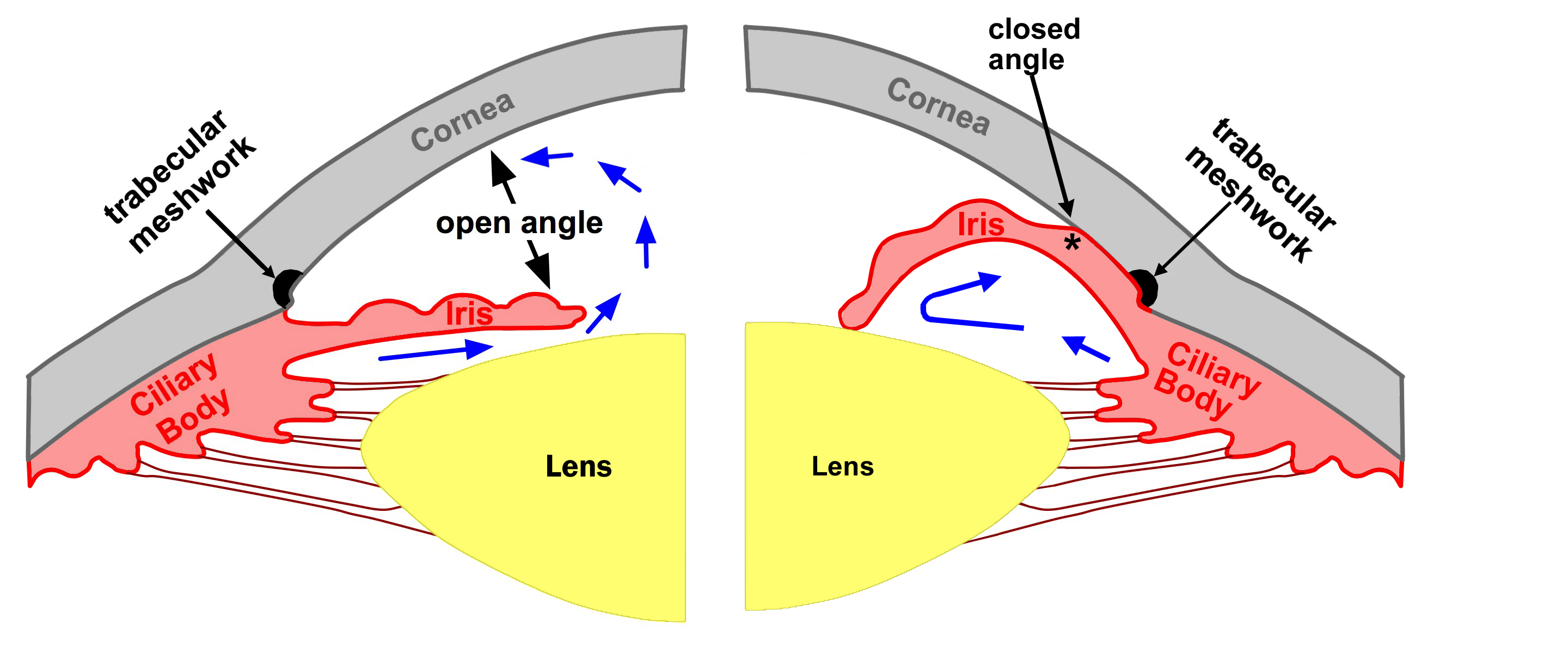
Figure 4-6. An open angle is shown on the left. Aqueous is free to pass between the iris and lens and drain through the trabecular meshwork. Pupillary block (angle closure) is shown on the right. Aqueous is trapped behind the iris which pushes it forward to obstruct the trabecular meshwork.
The mechanism of angle closure comes from a contact between the lens and iris. Once this contact occurs, aqueous fluid is unable to pass into the anterior chamber through the pupil. This is called pupillary block angle closure. (figure 4-6)
The aqueous fluid then accumulates behind the iris and pushes the iris forward. The peripheral iris then obstructs the trabecular meshwork or drainage angle. The inability for aqueous to exit the eye creates an IOP elevation. This process may occur acutely or chronically. If the pressure rise is sudden, pain occurs. When it happens chronically, it may not be symptomatic. Occasionally the episodes of angle closure may occur intermittently. In these cases, patients may have episodic symptoms of blurry vision, eye pain or headache.
The risk factors for angle closure glaucoma are not the same as in open-angle glaucoma. Those at risk tend to have narrow angles. Patients who are older, female, hyperopic (farsighted), or from an Asian background tend to be at risk for angle closure glaucoma. Age tends to be a risk factor because the lens thickens over time leading to a higher likelihood of pupillary block. Hyperopia tends to occur in people with small eyes. These eyes tend to be crowded and more likely to develop pupillary block (this will be covered in more depth in Chapter 5).
For those at risk, pupil dilation may also cause a higher likelihood of angle closure. Pharmacologic pupil dilation, therefore, is contraindicated in patients with narrow, occludable angles (figure 4-7). Various over-the-counter and prescription medications are also contraindicated due to the same mechanism. Once definitive treatment (laser peripheral iridotomy) is administered, these are no longer contraindicated
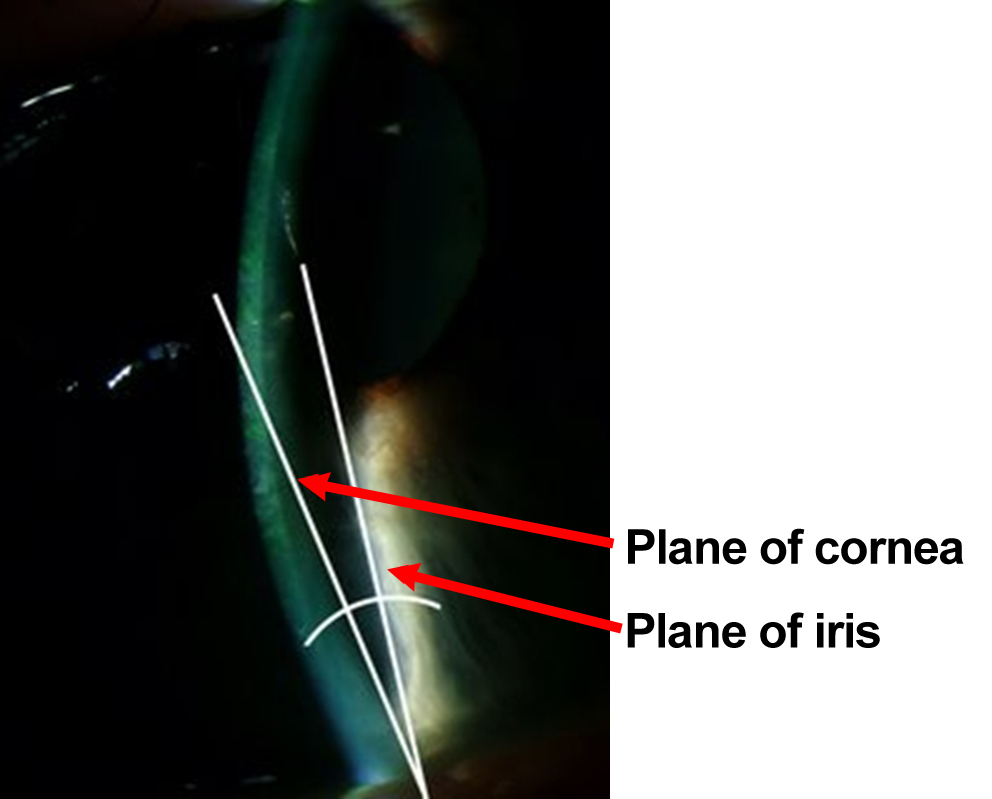
Figure 4-7. The angle between the iris and cornea is very narrow. Usually this angle is 45º. The angle in this picture is approximately 12°.
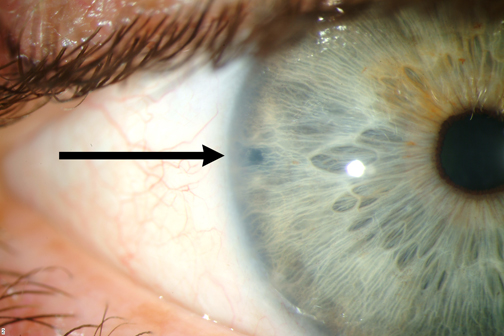
Figure 4-8. Laser Perpheral Iridotomy (LPI).
In acute angle closure glaucoma, a patient may have pain, nausea/vomiting, headache, a red eye, cloudy cornea, a shallow anterior chamber, and elevated IOP. Glaucoma eye drops are given to decrease the IOP but a definitive treatment needs to be done to break or relieve the pupillary block. The definitive treatment for acute angle closure glaucoma is laser peripheral iridotomy (LPI; figure 4-8). This laser procedure places a hole in the iris to circumvent the pupillary block, thereby allowing the iris to fall back and open up the drainage angle. If a patient cannot sit for a laser procedure, such as in children, a surgical iridectomy may be performed in the operating room. The same procedure (iridotomy) should eventually be performed in the unaffected fellow eye to prevent an occurrence in that eye. Once the procedure is performed in both eyes, pharmacologic pupil dilation or medications are no longer contra-indicated. Patients may be dilated or take these medications without fear of future acute angle closure glaucoma.
Section 4-E: Childhood Glaucoma
Although glaucoma occurs mostly in adults, children can get glaucoma as well. Glaucoma that is present at birth or within the first years of life is called congenital or infantile glaucoma. Congenital glaucoma occurs in 1 of 10,000 live births, so it is a rare condition. It may be inherited or can occur spontaneously without family history. It tends to occur more often in males and does not appear to have racial predilection.
As in adult glaucoma, an elevated IOP leads to glaucomatous optic nerve damage. The clinical presentation, however, is very different. Unlike in adult glaucoma, infants have eyes which are still growing and elevated intraocular pressure can cause a further increase in the size of their eyes. The enlargement of the eye from congenital glaucoma is called buphthalmos (meaning “ox eye”; figure 4-9). The high intraocular pressure can also cause their cornea to become cloudy or hazy, which can cause tearing and sensitivity to light (“photophobia”). One or both eyes may be affected.
Typical findings of congenital glaucoma include buphthalmos (enlarged eye), sensitivity to light, and tearing from the cloudy cornea. The elevated intraocular pressure also causes the posterior layer of the cornea to be torn (Haab's striae), in addition to optic nerve cupping and visual loss. In addition, these children are at high risk for developing “lazy eye” or amblyopia because the impaired vision secondary to cloudy cornea.
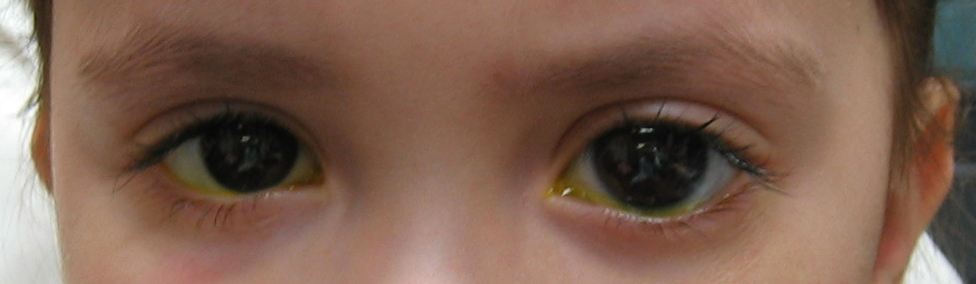
Figure 4-9. The left eye of this congenital glaucoma patient is noticeably larger than the right eye. The patient has buphthalmos of the left eye.
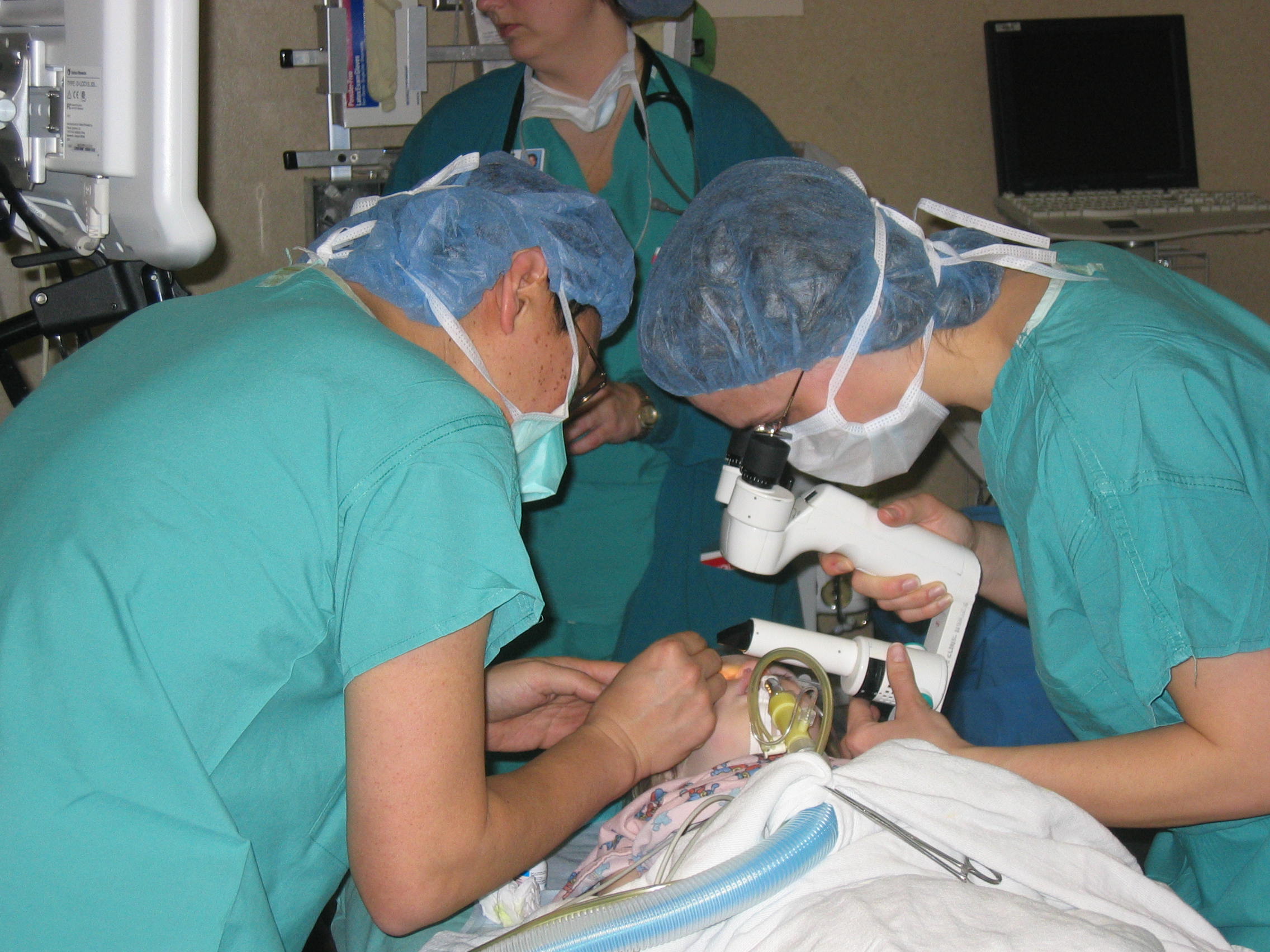
Figure 4-10. Infants with congenital glaucoma often require general anesthesia to perform an adequate examination.
The examination for children with suspected glaucoma includes measurements of the eye length and cornea, IOP, gonioscopy, as well as dilated examination for evaluation of the optic nerve. This may require that the infant be sedated (or put under general anesthesia) for examination. (Figure 4-10) Ultrasound is used for measurements of the eye length. Serial measurements may be made to determine if the eye is stable or becoming too large from high IOPs. Unfortunately, infants cannot verbalize their vision or perform visual field testing so their glaucoma is often followed with careful eye exams without visual field documentation.
Congenital glaucoma is caused by an abnormal insertion of the iris which leads to decreased outflow of aqueous fluid from the eye. Since this developmental abnormality is the cause of the high IOP, the treatment is aimed towards relieving this abnormality. The treatment is surgical incision of this abnormality which allows better outflow of aqueous fluid from the eye. This is in contrast to adult glaucoma where surgery is often the last line of treatment for glaucoma. In addition, once surgery relieves the elevated IOP, the optic nerve damage (or cupping) may be more reversible in contrast to irreversible nature of optic nerve damage in adult glaucoma. Despite treatment with surgery to relieve the anatomic abnormality, patients who have had congenital or infantile glaucoma need to be followed chronically for the progression of their disease. This means that they will still require periodic evaluation into adulthood. Childhood Glaucoma will be covered in more depth in Chapter 10.
Chapter 4. References
Alward WLM. Glaucoma: The Requisites in Ophthalmology. St Louis: Mosby, 2000.
Shields MB, Allingham RR, et al. Shields' textbook of glaucoma, 5th ed. Philadelphia: Lippincott Willliams & Wilkins, 2005.
Collaborative Normal-Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am J Ophthalmol. 1998;126(4):498-505.
Gordon MO, Beiser JA, Brandt JD, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, Parrish RK 2nd, Wilson MR, Kass MA. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120(6):714-30.
Robin AL, Pollack IP. Argon laser peripheral iridotomies in the treatment of primary angle closure glaucoma. Long-term follow-up. Arch Ophthalmol. 1982;100(6):919-23.