
Section 8-A: Glaucoma Laser Treatment
When medications fail to control glaucoma, laser and surgical treatment are often employed. Laser treatments for glaucoma can be used, depending upon the type of glaucoma and the anatomy of the drainage angle.
Section 8-A-1: Laser Trabeculoplasty
Laser trabeculoplasty delivers laser energy to the trabecular meshwork. The goal of treatment is to facilitate the outflow of aqueous humor from the eye in order to lower the intraocular pressure (IOP). The exact mechanism of lowering IOP is not completely understood. It is thought that the laser energy causes cellular changes within the drainage angle, which leads to increased aqueous outflow.
Certain patients have better results with laser trabeculoplasty than others. Patient selection is based on the type of glaucoma and trabecular meshwork pigmentation (coloring). There is no correlation between drainage angle pigmentation and the eye or skin color. Angle closure glaucoma is usually NOT treated with laser trabeculoplasty because the angle cannot be visualized for treatment. In addition, those with narrow angles may develop scarring (synechiae) between the cornea and iris with this type of laser treatment. Therefore, laser trabeculoplasty is usually reserved for open angle glaucomas. Patients with primary open angle, pigmentary, or pseudoexfoliation glaucoma tend to do well with laser trabeculoplasty. Some forms of open angle glaucoma, such as glaucoma from trauma or aphakia (previous cataract surgery without intraocular lens replacement), however, may not respond as well as primary open-angle glaucoma. In addition, laser trabeculoplasty is usually avoided in patients with active inflammation, as in uveitic glaucoma, since it can induce further inflammation.
The trabecular meshwork should be examined by gonioscopy (see Chapter 6, Section 6-C). More densely pigmented trabecular meshworks respond better to laser since pigment facilitates the uptake of laser energy (e.g., pigmentary and pseudoexfoliation glaucoma). Lightly pigmented trabecular meshworks may require more laser energy compared to densely pigmented trabecular meshworks, and may be less successful. The optimal candidate is a patient with an open angle with a densely pigmented trabecular meshwork. The eye should be quiet without inflammation. The pre-operative IOP should not be too high since one of the risks of laser trabeculoplasty is a post-operative IOP spike due to transient inflammation. Those with end-stage glaucoma may not be able to tolerate the potential post-laser IOP elevation, and instead may require a filtering surgery (see below).
Argon Laser Trabeculoplasty (ALT)
Argon laser trabeculoplasty (ALT) is a common glaucoma laser surgery, which is performed in an office setting. It uses an Argon laser which delivers energy in blue-green wavelengths (Figure 8-1). A topical glaucoma eye drop (e.g. brimonidine) is given 15 minutes prior to the procedure to prevent a post-laser IOP elevation. The procedure itself usually takes less than 10 minutes. Patients rarely experience discomfort during the procedure. Once the eye is anesthetized with topical eye drop, a contact lens is placed on the surface of the cornea (Figure 8-2). The gonioscopic contact lens allows visualization of the drainage angle. The laser spots are then delivered to the trabecular meshwork. Either 180º or 360º of the angle is treated per treatment session. Each area of the angle is treated only once to avoid scar formation in the angle.

Figure 8-1. Slit Lamp with Argon Laser used for laser trabeculoplasty. It may also be used for other ocular procedures including treatment for diabetic retinopathy.
Figure 8-2. Contact Lens used in Laser Trabeculoplasty.
After the laser procedure, another glaucoma eye drop is administered to prevent post-operative IOP spike. The IOP is measured 1 hour after the procedure to ensure that it is stable. Patients are advised to continue the glaucoma medications that they were using prior to the laser treatment. In addition, a short course of topical steroids is given to reduce post-operative inflammation. Patients return for follow-up examination in 4-6 weeks to determine if the ALT treatment decreased the IOP. If IOP is still uncontrolled, additional surgical intervention may be necessary.
Success rates of the ALT vary. Overall success rate is approximately 50% over 5 years. After this time, many patients require additional intervention to control their IOP.
Complications of ALT
The most common complication after ALT is the post-laser IOP spike. Fortunately, the pressure elevation is often transient and typically causes no long-term complication. This occurs from the inflammation that ensues after laser energy is delivered to the trabecular meshwork. It is treated with additional glaucoma medications as needed. Anterior segment inflammation (iritis) is another complication that may occur. Post-laser topical steroids are given to control inflammation. Topical steroids are used for several days to a week after treatment. Scarring between the iris and the angle (peripheral anterior synechiae) may develop after the laser, as well as changes to the peripheral cornea. These complications are uncommon in eyes with open angles, but may be more likely in narrow angles.
Selective Laser Trabeculoplasty (SLT)
Another glaucoma laser procedure is selective laser trabeculoplasty (SLT). This procedure utilizes a neodynium:YAG laser. Unlike ALT, SLT allows the surgeon to repeat the laser surgery over the same area of the angle, because the laser targets only the pigmented cells in the trabecular meshwork while sparing the non-pigmented cells from a thermal damage. Otherwise, the procedure is very similar to ALT. Either 180º or 360º of the angle is treated. It is performed in an office setting with administration of a glaucoma drop before and after the procedure, very similar to the ALT.
Potential complications of SLT are similar to ALT. There is the possibility of post-laser IOP elevation, which is minimized by the administration of glaucoma drops at the time of the laser treatment. An IOP check is performed 1 hour after the procedure. Patients are again advised to continue their previous glaucoma medications and are given a short course of topical steroids.
Section 8-A-2: Laser Peripheral Iridotomy (LPI)
Patients with narrow, occludable angles or who have an attack of acute angle closure glaucoma are treated with laser peripheral iridotomy (LPI). LPI is done to create a bypass channel for aqueous to flow from behind the iris to the front of the iris, and subsequently, into the drainage angle (see Chapter 4, Section 4-D). Prior to the advent of laser, a surgery was necessary to create this bypass (surgical iridectomy). Nowadays using laser energy, a hole is made in the peripheral part of the iris. The procedure is well-tolerated by patients under topical anesthesia. Several different lasers are utilized for this procedure including the Argon and YAG laser. Sometimes a combination of the two lasers is used to create the LPI. The Argon laser typically requires pigment for the uptake of the laser energy and therefore, is better for darker colored (i.e., brown) eyes. The YAG laser disrupts the tissue and is better suited for lighter colored (i.e., blue) eyes.
The procedure typically lasts 10-15 minutes. Prior to the procedure a glaucoma medication is given to prevent any post-laser IOP elevation. In addition, pilocarpine (cholinergic agent – see Chapter 7, Figure 8-3) is given to make the pupil smaller so that the hole can be placed peripherally. Administration of pilocarpine can temporarily give symptoms of a headache or brow ache.

Figure 8-3. Pilocarpine HCl 2% solution. Pilocarpine (a cholinergic agent) constricts the pupil prior to creation of a peripheral iridotomy.

Figure 8-4. Laser Iridotomy Contact Lens. Contact Lens used in Laser Trabeculoplasty.
In instances of acute angle closure glaucoma, the cornea may be too swollen and hazy which precludes a good view of the iris (see Chapter 9). Prompt administration of glaucoma medications, both topical and oral, helps to decrease the IOP. This can reduce corneal swelling and increase the clarity of the cornea. Glycerin is another agent which acts to draw fluid from the cornea and temporarily improve the corneal clarity. Once the view is adequate, the laser procedure can be performed. LPI is done under topical anesthesia. A special iridotomy contact lens (Figure 8-4) is placed on the cornea. The laser is then performed through the contact lens. After the procedure, another glaucoma medication is given. An IOP check is performed 1 hour after the laser treatment. Topical steroids are given for several days and then tapered or discontinued. This helps alleviate any inflammation from the laser. The inflammatory cell and pigments released during iridotomy may cause decreased vision after the laser, but this typically subsides in 3-4 days.
The LPI will relieve IOP elevation resulting from acute angle closure glaucoma (see Chapter 9). In cases of narrow, occludable angles with normal IOP, it will prevent acute angle closure from occurring in the future. Once the iridotomy is made, it remains open and the risk of acute angle closure glaucoma is eliminated. Even with patent iridotomy hole, patients may still develop IOP from other mechanisms. These patients are sometimes referred to have mixed mechanism glaucoma. The mechanism is mixed because there is a component of both closed and open angle contributing to the development of glaucoma.
Complications of LPI
The most frequent complication of LPI is a transient IOP increase. It is uncommon due to do the use of pre- and post-laser glaucoma medication (e.g. brimonidine). Ocular inflammation, or iritis, may also develop. This is treated with a short course of topical steroids. Ongoing inflammation may sometimes cause closure of the iridotomy site. Corneal or lens damage may occur with the laser treatment due to the proximity of these structures to the peripheral iris. In addition, retinal damage from the laser may rarely occur. Bleeding can occur during the LPI when iris blood vessels are disrupted during the laser procedure. It can be managed by applying gentle pressure with the iridotomy lens, or by simply waiting until blood coagulates on its own. Although rare, a significant amount of bleeding in the anterior chamber may cause the IOP to increase. Rarely, patients may complain of double vision (diplopia) or seeing a line in their vision. These visual aberrations may be from the iridotomy hole itself. The symptoms are often transient and become less noticeable over time.
Section 8-A-3: Laser Iridoplasty
After LPI relieves the pupillary block (Chapter 4, Section 4-D), a narrow angle will usually deepen when observed through a goniolens. If it does not, a patient may have a condition called plateau iris configuration. Plateau iris is caused by a forward rotation of the ciliary body, which causes narrowing of the drainage angle peripherally. Since the condition is not caused by pupillary block, the angle will not deepen after the LPI. To open the peripherally narrow angle, an iridoplasty (or gonioplasty) may be performed. This procedure uses Argon laser to shrink the peripheral iris, which pulls iris away from the trabecular meshwork and improves the aqueous outflow.
Iridoplasty is similar to the other laser procedures with regard to intra- and post-operative care. Topical anesthesia is used as well as a glaucoma medication (e.g. brimonidine) to prevent post-laser IOP elevation. A contact lens is used to visualize the peripheral iris and its contraction with Argon laser energy (Figure 8-5). The laser treatment is usually well-tolerated by patients. Another glaucoma drop is placed post-operatively and the IOP is checked 1 hour post-operatively to rule out IOP spike. A short course of topical steroids is prescribed. The complications are similar to those of the other ocular laser procedures.

Figure 8-5. Goldmann Contact Lens used for laser iridoplasty. This lens is the same as the one used for laser trabeculoplasty.
Section 8-B. Trabeculectomy: A filtering procedure
It is important to remember that glaucoma surgery is performed to lower intraocular pressure (IOP) in order to prevent further loss of vision, not to improve vision. Lowering the IOP to the target level helps to slow down or halt the progression of optic nerve damage and prevent further loss of vision. It is not possible to recover lost vision or existing optic nerve damage from glaucoma; vision loss from glaucoma is, therefore, irreversible. When medications or laser treatment fail to control the intraocular pressure (IOP) in glaucoma, the next step in the management of glaucoma often involves trabeculectomy, which is referred to as filtering procedure.
Preoperative Considerations
Trabeculectomy is a commonly performed glaucoma surgical procedure when medications fail to adequately control the IOP. It can be performed for most (but not all) types of glaucoma. It is necessary to have intact, non-scarred conjunctiva. The conjunctiva is a thin tissue that coats the surface of the sclera or eye wall. Trabeculectomy can be difficult to perform in an eye that has had previous ocular surgeries with scarred tissue. In this situation, the surgeon may elect to perform a glaucoma drainage tube implant (or seton) procedure (see next section).
Surgical Technique
The basic goal behind trabeculectomy is to create a small hole in the anterior chamber of the eye to allow drainage of the aqueous fluid toward the outside. (Figure 8-6) Trabeculectomy surgery starts with making an incision through the conjunctiva. The surgeon then creates a partial-thickness sclera flap (or trapdoor) on the sclera (eye wall). Underneath the scleral flap, a surgeon cuts a small hole into the anterior chamber, which allows the drainage of aqueous fluid through the scleral flap and into the sub-conjunctival space. An iridectomy (hole in the iris) is performed at this point to allow the scleral opening to stay open without being blocked by the iris tissue. The scleral flap is then tied down with stitches, that are loose enough to allow continuous drainage of the aqueous fluid. Finally, the overlying conjunctival tissue is closed with stitches to allow formation of a bleb or an elevation of conjunctival tissue formed by the aqueous fluid, which is being filtered out of the scleral flap (trapdoor) underneath. The filtering bleb is usually located in the superior aspect of the eye and covered by the upper lid (Figure 8-7). Consequently, it is not readily noticeable by a casual observer. The aqueous fluid from the filtering bleb is then slowly absorbed by the conjunctival and episcleral (on the surface of the sclera) blood vessels and drain into the orbital venous system.
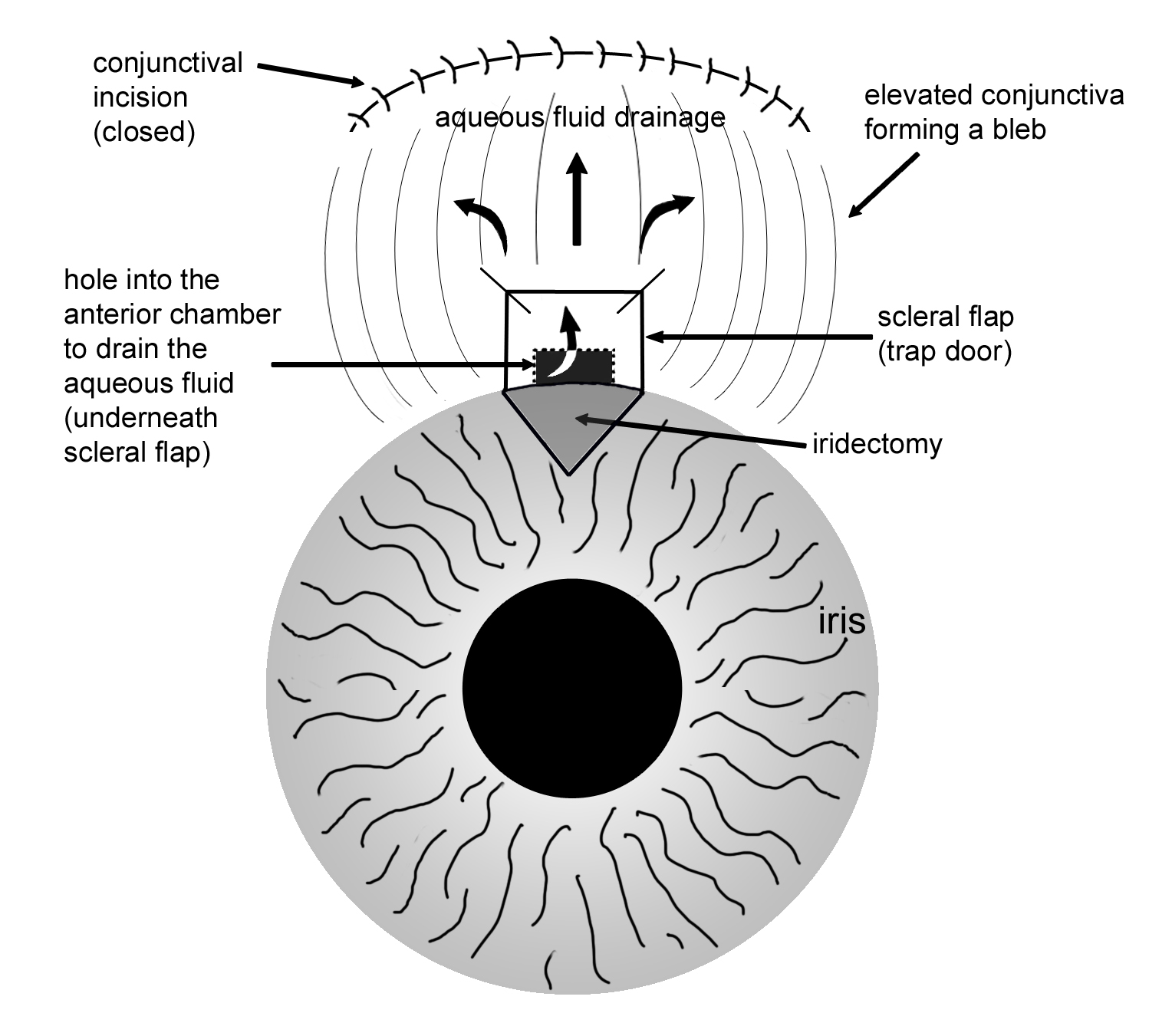
Figure 8-6. Illustration of a trabeculectomy surgery.
Starting in the late 1980’s, chemotherapeutic drugs (or anti-metabolite or anti-healing medications commonly used in cancer chemotherapy) have been used during or after trabeculectomy. These anti-metabolite drugs are used to decrease the amount of tissue healing following trabeculectomy. A large multi-center study has shown that 5-fluorouracil (an anti-metabolite medication) is effective in increasing the success rate of trabeculectomy. Anti-metabolites such as 5-fluorouracil and mitomycin C are widely used during trabeculectomy to increase the overall success of the surgery. On the other hand, the frequent use of the anti-metabolite medications during trabeculectomy can also increase the risk of hypotony (low intraocular pressure below the physiologic level), which is one of the complications of trabeculectomy (see below).
Anesthesia
The surgery uses a local anesthesia to the eye. Traditionally, a retrobulbar (behind the eye) anesthetic injection was used for local anesthesia. This involves injecting a small amount of anesthetic behind the eye under light intravenous sedation. Retrobulbar anesthesia works well not only in trabeculectomy but in many other types of ocular surgery. However, it can be occasionally associated with serious complications such as hemorrhage (bleeding) or even perforation of the eye. In certain cooperative patients, a topical (eye drop) anesthesia can be used to perform trabeculectomy rather than the retrobulbar injection anesthesia. The advantage of the topical anesthesia is quicker visual recovery and decreased risk associated with retrobulbar injection. The surgery usually takes less than an hour under local anesthesia and is done as an outpatient basis.
Postoperative Recovery and Follow-Up
Postoperatively, the recovery period is between 6-8 weeks. In trabeculectomy, the post-operative follow-up is particularly important because the success of the surgery depends on the rate and extent of conjunctival healing process. During this period, the surgeon follows the patient closely, usually on a weekly (sometime more frequent) basis initially. During follow-up visits, adjustments can be made to reduce the IOP if it is too high. This can be done by cutting (or pulling) stitches from the scleral flap with a laser to allow additional filtration (laser suturelysis, Figure 8-8). Occasionally, the surgeon may elect to needle the bleb post-operatively if there is excessive conjunctival scarring process. A small gauge needle is used to break up the scar tissue to allow more filtration of the aqueous fluid, and usually performed in a minor procedure room under topical anesthesia. If the IOP is too low, the surgeon may reduce the amount of anti-inflammatory (or steroid) medications to allow additional healing process. In short, there are many adjustments that may need be done postoperatively to maximize the chance of surgical success. Thus, it is very important for the patient to have a proper postoperative follow-up under the direction of the treating surgeon.
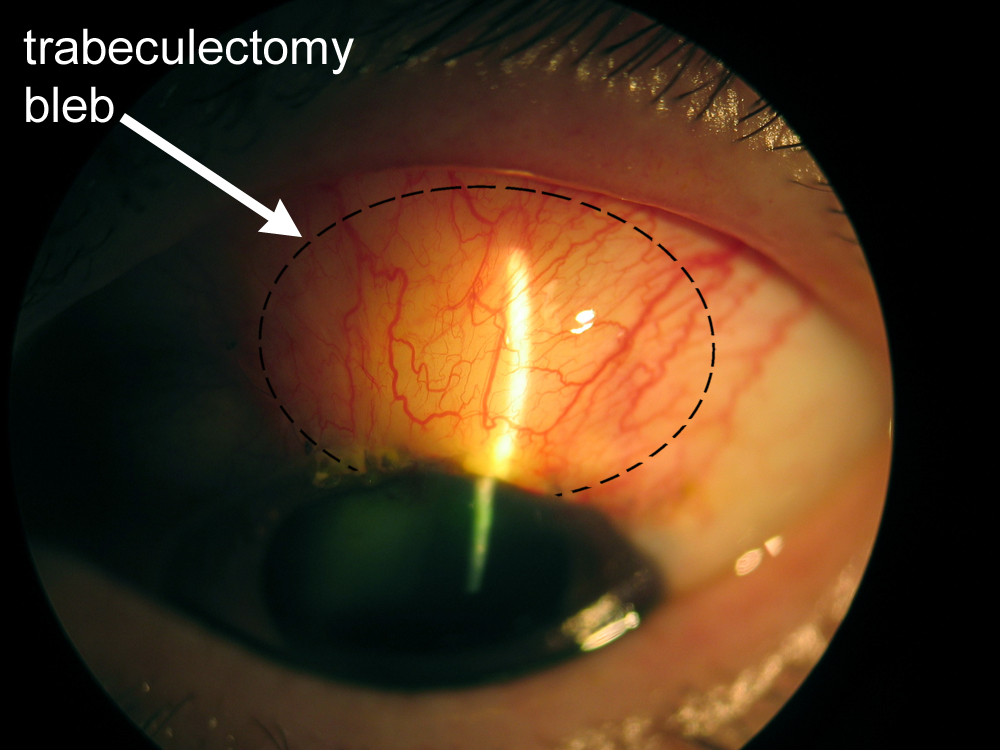
Figure 8-7. Trabeculectomy filtering bleb.
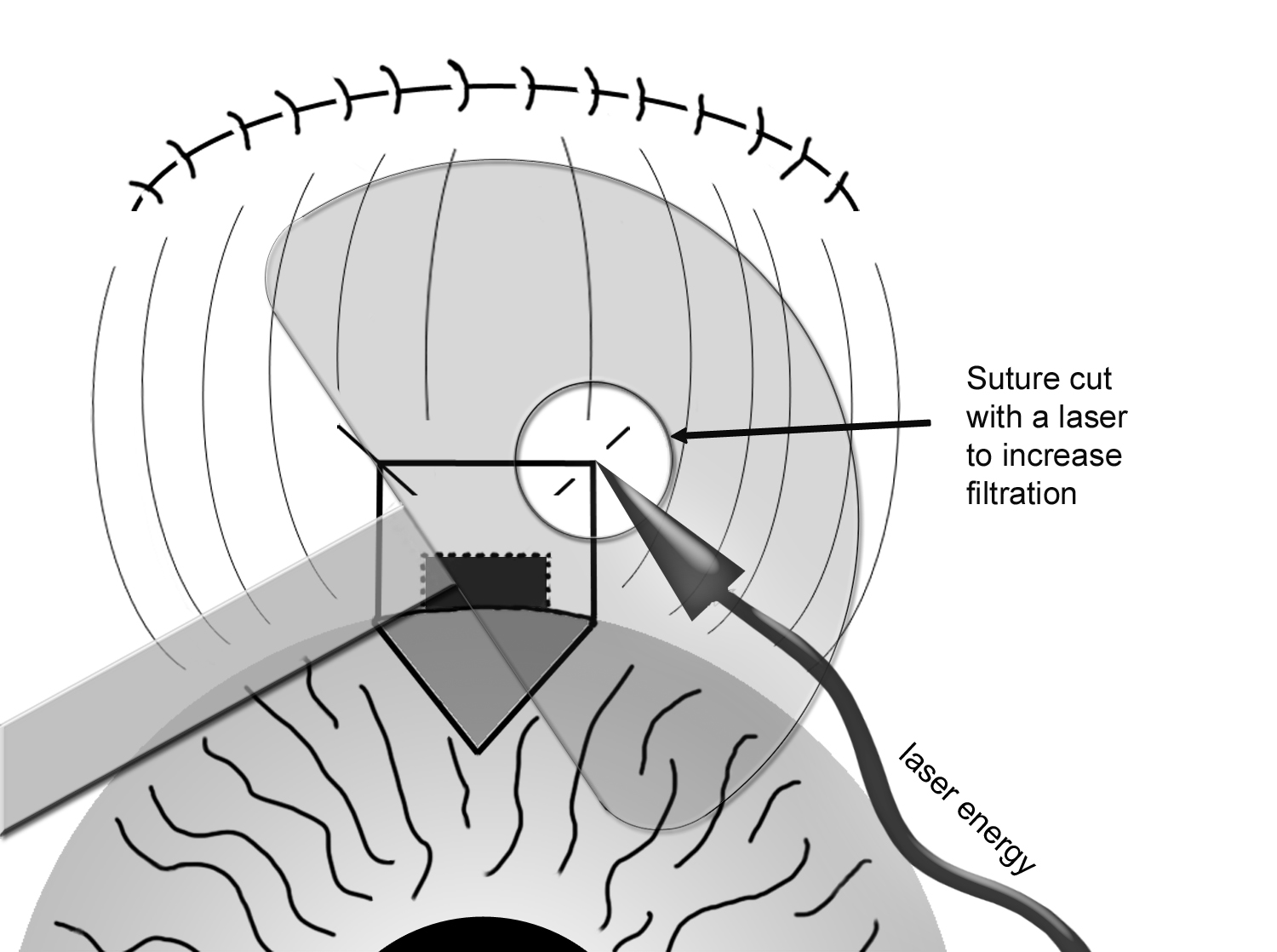
Figure 8-8. Postoperative Laser Suturelysis to increase filtration of trabeculectomy
Success and Complications of Surgery
The success rate of a trabeculectomy is approximately 65-70%. Additional 20% can have a qualified success, meaning the goal IOP is achieved using one or more anti-glaucoma medication(s) postoperatively. Approximately 10-15% of the trabeculectomy may fail in the first few months due to excessive conjunctival scarring. In approximately 1% of the time however, there can be more serious complications such as hemorrhage (bleeding), infection, and other complications. Depending on the complication, it may result in temporary or even permanent reduction in vision. It may also require further surgical procedure to correct the complication. It is important to discuss these potential complications with the treating physician before surgery.
The most common short-term complication of trabeculectomy surgery is that it may fail to adequately lower the IOP. This may occur due to the excessive scarring of the conjunctival tissue with decreased filtration of the aqueous fluid out of the eye. On the other hand, the IOP may be too low (called hypotony) due to the excessive filtering of the aqueous fluid or leaking wound. Low IOP (typically below 5 mmHg) can cause blurry vision and can be associated with shallowing of the anterior chamber, cataract formation, and greater risk of intraocular fluid accumulation (choroidal effusion) or intraocular bleeding (suprachoroidal hemorrhage). The suprachoroidal hemorrhage is a particularly feared complication after trabeculectomy, because it is often associated with pain, elevated IOP, and permanent decrease in vision. It often requires additional surgery to drain the blood.
There are also long-term complications of having a trabeculectomy bleb around the eye. If there is a leak from the bleb, the IOP may become too low. In addition, the bleb leak can increase the risk of infection. An infection in a post-trabeculectomy eye can be serious because of the surgical hole in the sclera may allow a direct access by the offending micro-organism to the inside of the eye. Such intraocular infection can seriously compromise the vision and even integrity of the eye itself. Therefore, any symptoms of infection in a post-trabeculectomy eye such as pain, decreased vision, redness, and purulent discharge, should be reported and examined promptly. This may occur even years after the surgery. For this reason, post-trabeculectomy patients are encouraged to always wear goggles during swimming and are discouraged from wearing contact lenses in order to decrease the possibility of a bleb infection (called blebitis or bleb-associated endophthalmitis).
Even a successful trabeculectomy surgery may not last forever; the surgery is considered successful if it controls the IOP for a period of 7-8 years. If the first trabeculectomy fails, it can be repeated second (rarely third) time to control glaucoma. Subsequent trabeculectomies usually have a higher chance of failure than the primary trabeculectomy surgery. If trabeculectomy fails to control glaucoma adequately, the surgeon may consider a glaucoma drainage tube (or seton) (see next).
Section 8-C. Glaucoma Drainage Devices (Glaucoma Tube or Seton Implant)
A glaucoma drainage device (GDD) or tube is usually implanted when glaucoma is uncontrolled with medications, laser, or trabeculectomy. It is also used when there is not enough healthy tissue to proceed with trabeculectomy or in cases where trabeculectomy would likely fail. The goal of GDD is to allow aqueous fluid to leave the eye so that IOP will be lowered and halt the progression of glaucomatous visual loss.
Several models of GDD exist. The implants are typically made of silicone or polypropylene. Ahmed (figure 8-9. New World Medical, Inc., Rancho Cucamonga, CA), Krupin (Hood Laboratories, Pembroke, MA), Baerveldt (figure 8-10. Advanced Medical Optics, Inc., Santa Ana, CA), and Molteno (IOP Inc., Costa Mesa, CA) implants are the most common implants available. The GDDs are tubes, which are attached to a plate, and allow aqueous fluid to drain from the inside to outside of the eye. Both the tube and plate are covered by donor tissue and by patient’s own conjunctiva. A GDD can be with or without a valve. The tubes with a valve (Ahmed and Krupin) are made so that a set pressure is required before the tube opens and begins to drain aqueous fluid. They are usually implanted in cases where an immediate lowering of IOP is desired. The tubes without a valve (Baerveldt and Molteno) have no resistance to outflow of aqueous fluid, which may result in too low of an IOP (hypotony) initially. Severe or prolonged hypotony may lead to decreased vision or hemorrhage. To avoid these complications, the non-valved tube is often tied off with a dissolvable suture. The suture dissolves in approximately 6 weeks. During this time period, scar tissue develops over the tube plate which causes the required resistance to outflow that is necessary to avoid hypotony. Patients with non-valved GDDs are forewarned that the vision may suddenly become blurry with floaters about 6 weeks after surgery when the tube opens. The symptoms typically resolve over time.

Figure 8-9. Ahmed implant.
Figure 8-10. Baerveldt 350 implant.
Indication for Tube Surgery
Drainage implant surgery is used in patients who may have failed other filtering surgeries and need a lower IOP. It is also useful in patients at higher risk of failure from trabeculectomy, due to previous scarring or inflammation of conjunctival tissue. Such conditions include neovascular glaucoma, uveitic (inflammatory) glaucoma, and conjunctival scarring from previous ocular surgeries. In these cases, GDD surgery may be more successful in controlling glaucoma.
The Tube Surgery
Prior to surgery, patients may be asked to stop any medication that “thins” the blood (e.g. aspirin, ibuprofen, warfarin, (Coumadin), clopidogrel (Plavix), or ticlopidine (Ticlid)). Usually, this is coordinated with the primary care physician who is managing these medications. The surgery is typically performed under local anesthesia. A periocular injection of anesthetic is given to provide adequate pain control.
The conjunctiva in the designated quadrant of the eye is opened so that the eye muscles may be identified. The plate of the tube is placed between or underneath the eye muscles of the eye. It is fastened to the underlying sclera with permanent sutures. The tube is then cut to appropriate length and inserted into the anterior chamber. A small piece of donor tissue (sclera, cornea, pericardium, or dura) is then placed over the tube so that it does not erode through the overlying conjunctiva. The conjunctiva is placed back into place over the plate to cover the tube. In non-valved tube, a dissolvable suture may be used to tie off the tube. The surgery is typically performed under local anesthesia, in an outpatient setting.
Post-operative care
Patients who receive a valved implant are asked to discontinue their glaucoma medications after surgery, since the tube is expected to lower IOP immediately. During the postoperative course, glaucoma medications may be re-instituted based upon the level of IOP. Since non-valved implants do not work until approximately 6 weeks after surgery when the suture dissolves, patients are often asked to continue their glaucoma medications until this occurs. Once the tube opens and the IOP decreases, medications are discontinued as tolerated. In both types of implants, patients are treated with topical steroids and antibiotics post-operatively. Occasionally pupil-dilating drops (cycloplegics) are used to keep the eye comfortable and to keep the anterior chamber well-formed.
Patients are asked to avoid heavy lifting, bending their head down below the waist, and getting dirty water in the eye. A shield is often used at night for eye protection initially. The postoperative visits are important since medication adjustments will be made after the IOP is checked. It is important not to dwell too much on the IOP in the early postoperative period since it can fluctuate significantly. It is not uncommon to have significant fluctuation in IOP during the first several weeks after glaucoma surgery.
Success Rates
In general, the success rate of GDD is approximately 75% after 1-2 years. This varies slightly according to the type of GDD used and type of glaucoma being treated. Another 10-15% are successful with the addition of glaucoma medications (partial success). Failure to control IOP occurs in approximately 10%. These patients will need further surgical intervention to control their glaucoma.
Complications
Any ocular surgery including the GDD has the potential complication of bleeding, infection, and discomfort. After GDD surgery, vision loss may occur from bleeding, infection, retinal detachment, swelling of the cornea or retina, hastening of cataract formation, or too low an eye pressure (hypotony). Additional glaucoma medications or surgery may be needed if the IOP remains higher than desired. The implant may also migrate or become exposed by eroding through the conjunctival tissue. Sometimes, the eyelid may become droopy (ptosis) after surgery or double vision (diplopia) may occur. These complications can be treated medically or surgically. Serious, vision-threatening complications are uncommon. If they occur, additional medication or surgery may be needed.
Section 8-D. Ciliary body ablation (cycloablation)
When trabeculectomy or glaucoma drainage tube (seton) has failed to control glaucoma, then the treating physician may consider cycloablation (ablation or destruction of the ciliary body which produces the aqueous fluid). Because cycloablation involves permanent destruction of the ciliary body, it is usually the last line of treatment for uncontrolled glaucoma. Before the advent of laser, this was done using a cryoprobe (freezing probe) to freeze the ciliary body (cyclocryotherapy). This was often an uncomfortable procedure and was also associated with significant complications, including inflammation and loss of vision. Starting in the 1990’s, cyclocryotherapy was largely replaced by a laser procedure called CycloPhotoCoagulation or CPC). A portable diode laser is used to perform CPC under a local (retrobulbar) anesthesia as an outpatient surgery (Figure 8-11). CPC usually takes less than 30 minutes to perform, including the anesthesia.
Cyclophotocoagulation (CPC) is a useful procedure for a refractive glaucoma which cannot be controlled by medications and other surgeries. The success rate for CPC is in the range of 60-70%, and it can be repeated if needed. The recovery period is usually 4-6 weeks. The follow up visits are not as intensive as the filtering surgery, and this may offer advantage to some patients. Post-operatively, the eye is treated with tapering regimen of anti-inflammatory steroids. There are a number of potential complications associated with CPC, although less than those of cyclocryotherapy. Because CPC can be associated with decrease in vision post-operatively, CPC is commonly reserved for patients who already have reduced vision from either glaucoma or other causes pre-operatively. CPC is also associated with increased inflammation, bleeding, and hypotony (low IOP usually below 5 mmHg). Hypotony is a particularly feared complication of CPC because it is often difficult to raise the IOP after a permanent destruction of the ciliary body. Fortunately, it occurs more rarely with CPC than cyclocryotherapy.

Figure 8-11A. Diode Cyclophotocoagulation (CPC) G-Probe
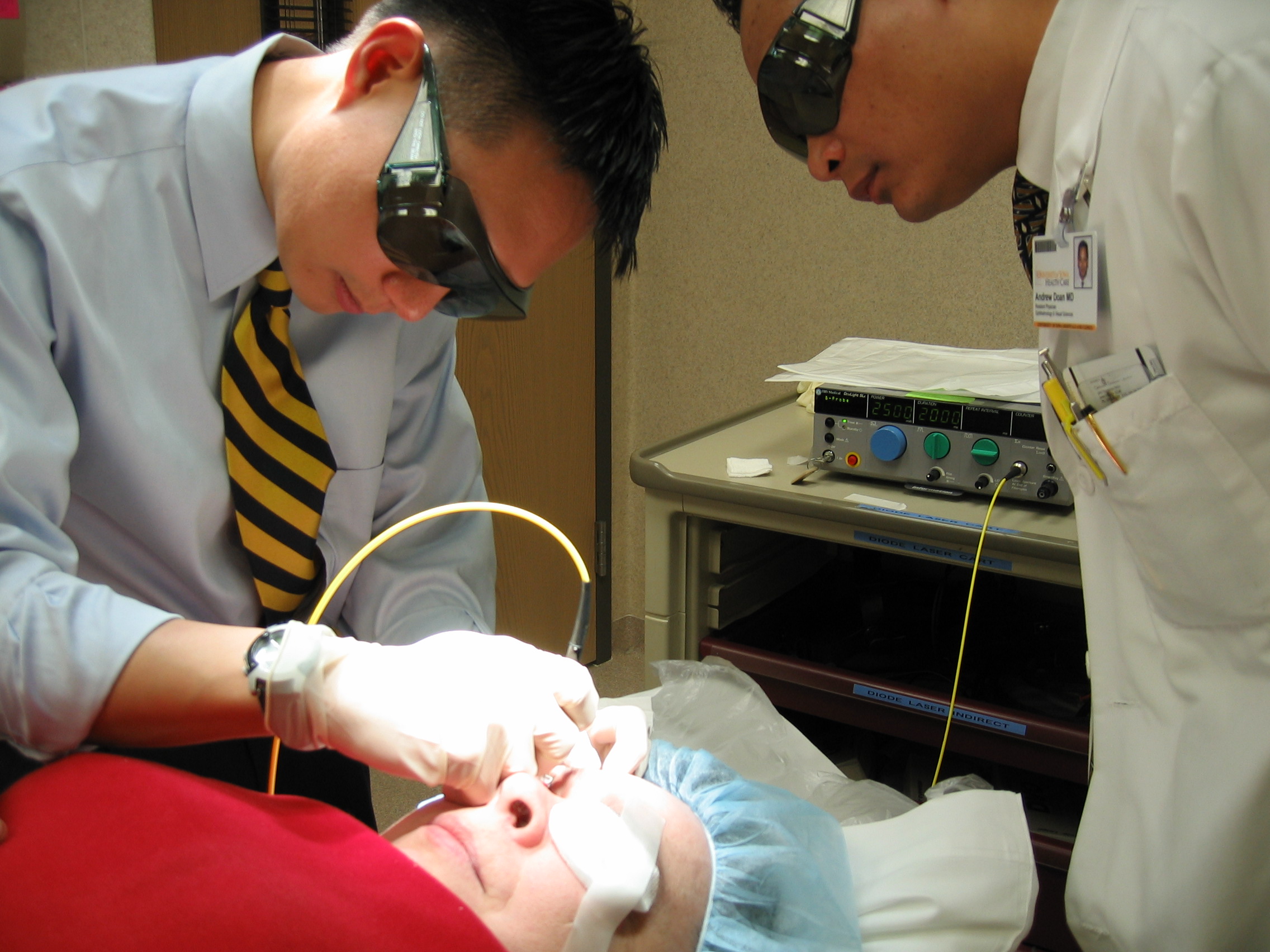
Figure 8-11B. CPC Procedure being performed on the right eye of a glaucoma patient
Chapter 8. References
Allingham RR, Damji K, Freedman S, Moroi S, Shafranov G. Ch 40: Filtering Surgery. In: Shield’s Textbook of Glaucoma. 5th Ed. Lippincott Williams and Wilkins, Philadelphia, p.568-609, 2005.
Allingham RR, Damji K, Freedman S, Moroi S, Shafranov G. Ch 41: Drainage Implant Surgery. In: Shield’s Textbook of Glaucoma. 5th Ed. Lippincott Williams and Wilkins, Philadelphia, p. 610-625, 2005.
Allingham RR, Damji K, Freedman S, Moroi S, Shafranov G. Ch 43: Cyclodestructive Surgery. In: Shield’s Textbook of Glaucoma. 5th Ed. Lippincott Williams and Wilkins, Philadelphia, p.644-661, 2005.
Alward, WLM. Ch 17: Incisional Surgical Treatment. In Glaucoma: The Requisites in Ophthalmology, Mosby, St. Louis, p. 214-239, 2000.
Alward, WLM. Ch 18: Cyclodestructive Procedures. In Glaucoma: The Requisites in Ophthalmology, Mosby, St. Louis, p. 240-245, 2000.
Ayyala RS, Zurakowski D, Smith JA, et al. A clinical study of the Ahmed glaucoma valve implant in advanced glaucoma. Ophthalmology 1998;105:1968-76.
Latina MA, Sibayan SA, Shin DH, et al. Q-switched 532-nm Nd:YAG laser trabeculoplasty (selective laser trabeculoplasty): a multicenter, pilot, clinical study. Ophthalmology 1998;105:2082-8.
Lloyd MA, Baerveldt G, Heuer DK, et al. Initial clinical experience with the Baerveldt implant in complicated glaucomas. Ophthalmology 1994;101:640-50.
Rosman M, Aung T, Ang LP, et al. Chronic angle-closure with glaucomatous damage: long-term clinical course in a North American population and comparison with an Asian population. Ophthalmology 2002;109:2227-31.
Shingleton BJ, Richter CU, Dharma SK, et al. Long-term efficacy of argon laser trabeculoplasty: a 10-year follow-up study. Ophthalmology 1993;100:1324-9.
Weiss HS, Shingleton BJ, Goode SM, et al. Argon laser gonioplasty in the treatment of angle-closure glaucoma. Am J Ophthalmol 1992;114:14-8.