
Section 5-A: Risk Factors for Primary Open Angle Glaucoma
Primary open angle glaucoma (POAG) has several well-known risk factors. They include elevated intraocular pressure (IOP), older age, black race and positive family history.
Elevated IOP: While elevated IOP is not necessary for having glaucoma (for example, normal tension glaucoma), it is clear that elevated IOP increases the chance of developing glaucoma (Table 5-1). For example, if you have an IOP of 30 mmHg, your chance of having glaucoma is 25.4%, and your risk of developing glaucoma is 39 times higher than those with IOP <15mmhg.
| IOP (mmHg) | Prevalence in population (%) | Relative Risk (fold) |
|---|---|---|
| <15 | 0.7 | 1.0 |
| 16-18 | 1.3 | 2.0 |
| 19-21 | 1.8 | 2.8 |
| 22-24 | 8.3 | 12.8 |
| 25-29 | 8.3 | 12.8 |
| 30-34 | 25.4 | 39.0 |
| ≥35 | 26.1 | 40.1 |
We now know that measurement of IOP is at least partly dependent on the corneal thickness. In thicker corneas, the true IOP is lower than the measured IOP; in thinner corneas, the true IOP is higher than the measured IOP. Therefore, the measurement of corneal thickness is an important part of measuring IOP (Fig. 5-1).

Figure 5-1. A patient undergoes corneal thickness measurement (pachymetry)
Older age: Like many other diseases, glaucoma occurs more frequently in older population (Table 5-2). For example, the prevalence of POAG increases from 0.6% in a 40-year old to 7.3% in an 80 year-old white person. This is > 12 fold increase in risk in the 40-year span.
| Age (years) | White | Black | Hispanic | Asian |
|---|---|---|---|---|
| 40-49 | 0.6 | 1.2 | 0.5 | 0.3 |
| 50-59 | 1.5 | 4.1 | 1.6 | 1.6 |
| 60-69 | 2.7 | 5.5 | 1.7 | 1.8 |
| 70-79 | 5.1 | 9.2 | 5.7 | 2.9 |
| 80 | 7.3 | 11.3 | 12.6 | N/A |
| Overall | N/A | 4.7 | 2.0 | 1.2 |
Black race: The prevalence is generally higher in blacks than in whites (Table 5-2). Hispanics had similar prevalence as whites, except for the oldest age group (80 years) whose prevalence (12.6%) was comparable to that of blacks. Asians had similar prevalence as whites. Blacks are not only more likely to develop glaucoma, they are also more likely to lose sight and become blind once glaucoma develops.
Positive family history of glaucoma: Like many other diseases, there is hereditary component to glaucoma. If you have a first-degree relative (parent, sibling or child) with glaucoma, your risk of developing glaucoma is 2-4 times higher than those without family history. This strongly suggests genetic component to the disease. Several genes (and their location in the chromosome or DNA) have been implicated in POAG. The most well-studied is the Myocilin gene on chromosome 1, which accounts for 3-5% of POAG patients. Other, yet to be discovered, genes are suspected to play a role in the remaining POAG population.
Additional minor risk factors for POAG include: myopia (near-sightedness), diabetes, and elevated blood pressure. While elevated blood pressure is associated with the increase in the risk of POAG in a population study, there is no direct relationship between the two in individual patients. In other words, the IOP and blood pressure are not directly correlated (linked) in a given patient. Migraine headaches and Raynaud’s disease (poor circulation in hands and feet) have been associated with normal tension glaucoma.
Section 5-B: Risk Factors for Primary Angle-Closure Glaucoma (PACG)
The primary angle-closure glaucoma (PACG) has several risk factors of its own. They include: older age, Asian race, female gender, hyperopia (far-sightedness), and positive family history.
Older age: Like POAG, the older age increases the risk of PACG. One reason for this may be that as you get older, cataract (cloudy lens) develops which results in thickening and narrowing of the anterior chamber drainage angle (see Chapters 3 for the eye anatomy). The narrow drainage angle predisposes the patient for PACG.
Asian race: The Asian populations are at a higher risk for developing PACG than the other races (see Chapter 2: Table 2-3). PACG exists in 0.3 ~ 2.7% of the adult Asian populations, while it exists in only 0.1 ~ 0.6% in other races (white, black and Hispanic adult population). The exact reason for this is unclear. However, smaller anterior chamber depth has been found to be associated with PACG (Fig. 5-2). It is possible that the Asian population as a group has smaller anterior chamber depth than other populations, thus increasing the risk for PACG.
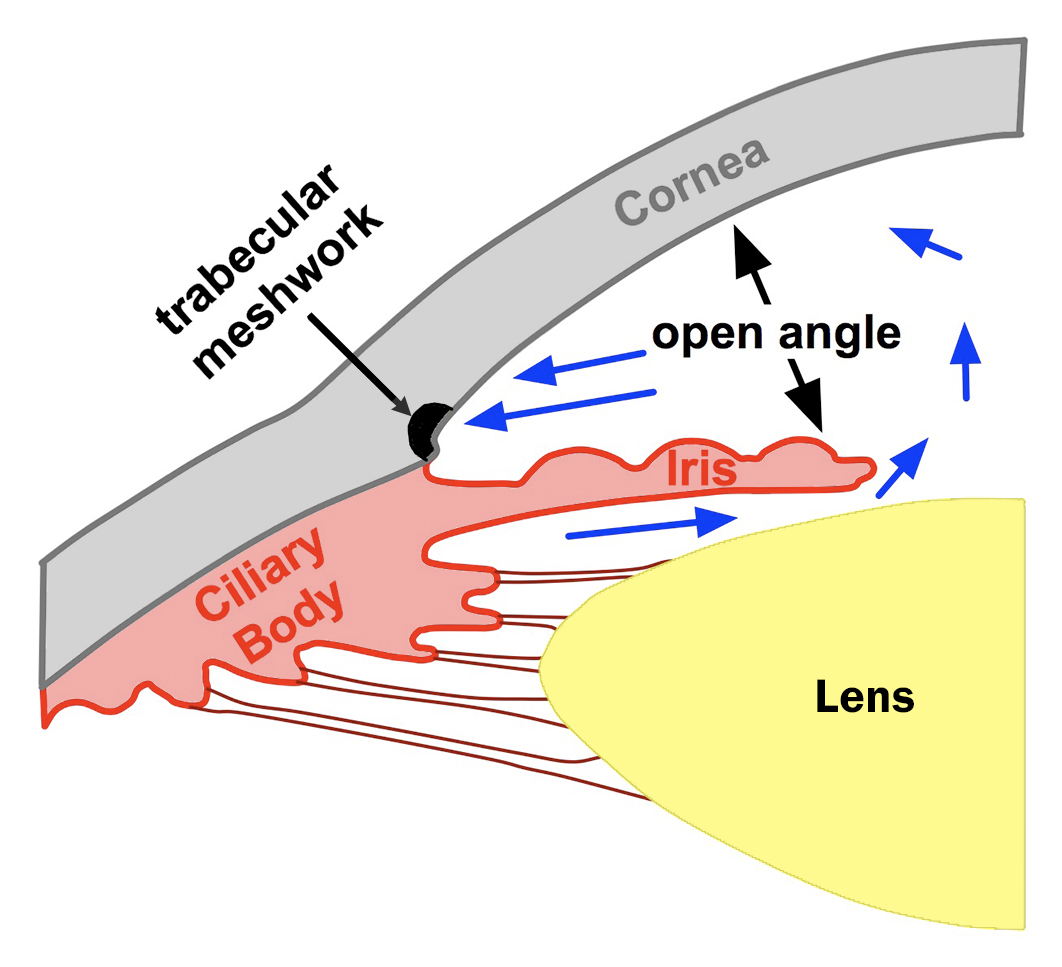
Figure 5-2A. The drainage angle formed between the cornea and the iris is wide open to about 40˚. Aqueous fluid flows freely to the trabecular meshwork and out of the eye. Note the anterior chamber depth is large.
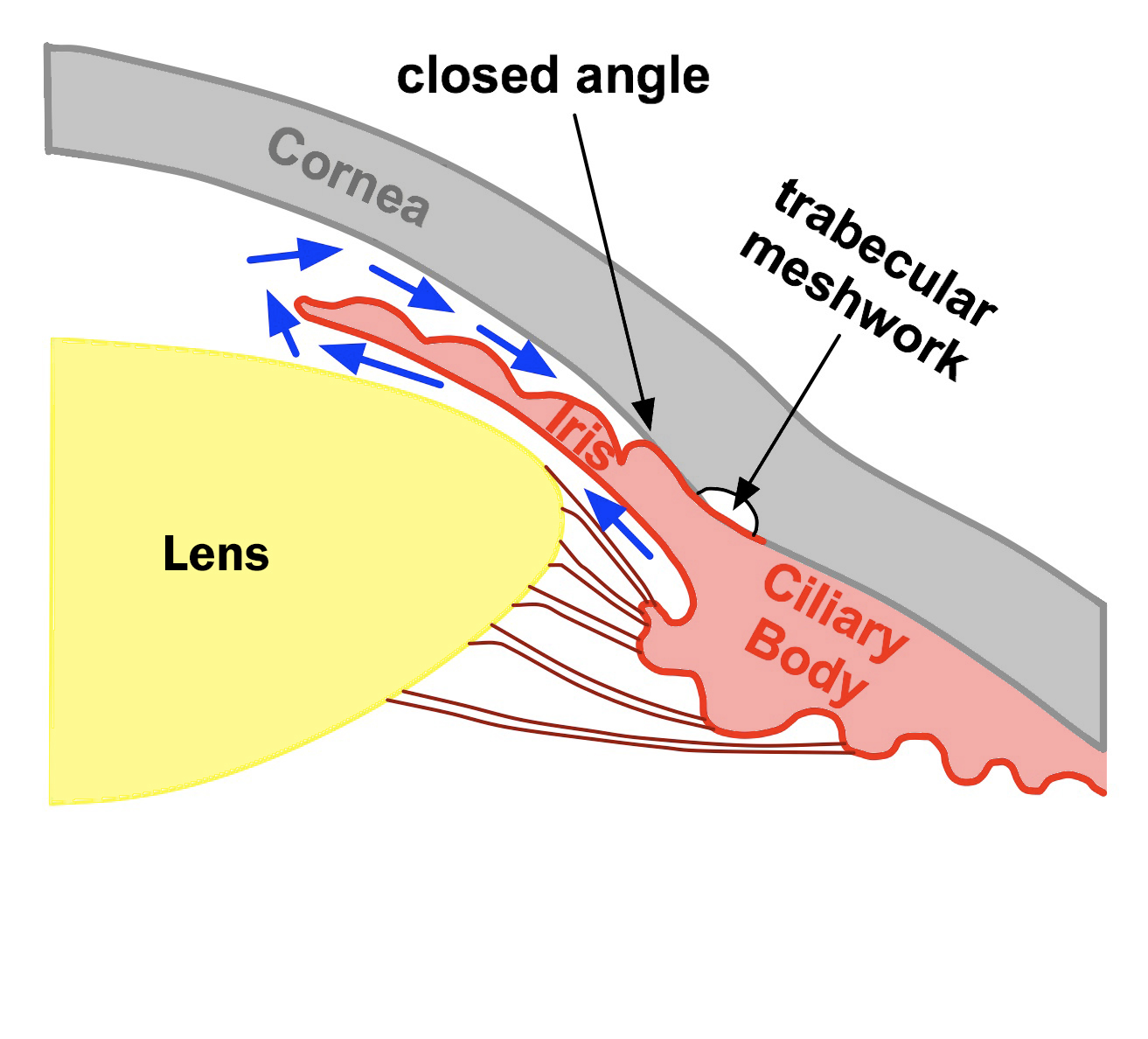
Figure 5-2B. The drainage angle is closed. The iris and the cornea are in contact and block the flow of aqueous fluid to the trabecular meshwork. The fluid is trapped in the eye causing intraocular pressure to rise. Note the anterior chamber depth is small.
Female gender: In PACG, females are more common than males. It is generally believed that females (compared to males) have smaller eyes and therefore, smaller anterior chamber and narrower drainage angle. These are in turn anatomical risk factors for development of PACG.
Hyperopia (far-sightedness): Like female gender, far-sighted people tend to have smaller eyes compared to near-sighted people. Smaller eyes tend to have smaller anterior chamber depth and narrower drainage angle, increasing the risk of PACG.
Positive family history of PACG: It has been reported that up to 20% of relatives of PACG patients have anatomically narrow drainage angle. It is generally believed that the eye size (like height) is inherited. If the small eye size (and therefore, narrow drainage angle) is inherited, this would explain the inheritability of PACG.
It is important to identify those who are at risk for development of PACG, because the rate of blindness from angle-closure glaucoma is higher than in POAG. There are studies in Asia looking at best ways to screen for PACG in a large population. Until we know better ways to detect PACG early, our recommendation is to seek periodic ophthalmic evaluation by an eye doctor (Table 5-3).
| Age group | No glaucoma risk factors | With glaucoma risk factors |
|---|---|---|
| 20-29 | At least once during interval | Every 3-5 years |
| 30-39 | At least twice during interval | Every 2-4 years |
| 40-64 | Every 2-4 years | Every 2-4 years |
| 65 | Every 1-2 years | Every 1-2 years |
In those patients who are susceptible for PACG (or have narrow drainage angles), taking certain medications (both prescription and over-the-counter) can precipitate an acute angle closure glaucoma attack. These medications contain ingredients that tend to dilate the pupil (as a side-effect). In susceptible patients with a narrow drainage angle (called an occludable angle), the pupil dilation can precipitate an acute angle-closure attack, often raising the IOP to very high levels (40-50 mmHg are not uncommon). It is important to ask the pharmacist and read the drug label thoroughly before taking these medications. If you are not sure, ask your eye doctor whether you are at risk for development of angle-closure glaucoma before taking the medication. Finally, several classes of drugs have been reported to cause acute angle closure, even in non-susceptible patients. For example, topiramate (Topamax) used in seizure and migraine disorders has been reported to cause acute angle closure glaucoma in patients who are typically not at risk. Other medications associated with angle closure glaucoma include tricyclic anti-depressants, serotonin-uptake inhibitors, and diuretics. It is important to monitor for glaucoma if you are taking one of these medications.
5-C. Risk factors for childhood glaucoma
Childhood glaucoma usually refers to those that occur very early in life (usually under the age of 2 years). It is also commonly referred to as infantile or congenital glaucoma (see Fig 5-3). When glaucoma occurs between the ages of 4 - 35 years, it is usually referred to as “juvenile” glaucoma.
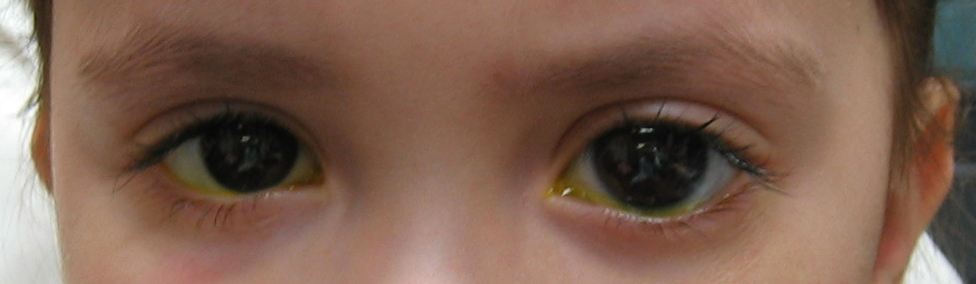
Figure 5-3. This young girl has congenital glaucoma in her left eye, which is noticeably larger than the right eye due to increased intraocular pressure.
Infantile glaucoma is a rare disease; it occurs in approximately 1 in 10,000 live births. Infantile glaucoma can occur in isolation, or along with other ocular or systemic abnormalities (for more detail, see Chapter 10). When it occurs in isolation, it is called primary infantile (or congenital) glaucoma (Figure 5-3). Primary infantile glaucoma occurs more commonly in boys than in girls (at the ratio of 3:2); however in Japan the gender ratio may be reversed (2:3 for boys:girls). While most of them occur without family history, approximately 10% have positive family history. If the first child has the disease, the risk of disease for the second child is about 3%. If the first 2 children have the disease, the risk for the third child is up to 25%. Thus, all siblings of an affected child should be examined closely by a pediatric ophthalmologist. Mutations in the CYP1B1 gene on chromosome 2 has been found to be associated with congenital glaucoma. In the future, we may discover additional genes responsible for congenital glaucoma.
Juvenile glaucoma presents between the ages of 4 and 35, and usually associated with very high IOP. They typically have very strong positive family history of glaucoma with several older members of the family having been affected by the disease. Mutations in the gene, Myocilin (sometimes called TIGR) on chromosome 1, have been found to be associated with patients with juvenile glaucoma. If you have a strong positive family history of infantile or juvenile glaucoma, it would be reasonable to be screened for glaucoma by an eye doctor at a younger age.
Chapter 5. References
Allingham RR, Damji K, Freedman S, Moroi S, Shafranov G. Clinical Epidemiology of Glaucoma In: Shield’s Textbook of Glaucoma. 5th Ed. Lippincott Williams and Wilkins, Philadelphia, p170-190, 2005
Allingham RR, Damji K, Freedman S, Moroi S, Shafranov G. Pupillary-Block Glaucomas In: Shield’s Textbook of Glaucoma. 5th Ed. Lippincott Williams and Wilkins, Philadelphia, p217-234, 2005
Kwon YH, Caprioli J: Primary Open Angle Glaucoma. In: Duane’s Clinical Ophthalmology, Tasman W, Jaeger EA, eds.; J.B. Lippincott Co., Philadelphia Chapter 52:1-30, 1999
Allingham RR, Damji K, Freedman S, Moroi S, Shafranov G. Molecular Genetics of Glaucomas In: Shield’s Textbook of Glaucoma. 5th Ed. Lippincott Williams and Wilkins, Philadelphia, p163-169, 2005
Alward, WLM. Glaucoma: The Requisites in Ophthalmology, Mosby, St. Louis, 2000