
The purpose of glaucoma treatment is to halt or slow down the progression of nerve damage. Because the optic nerve damage from glaucoma is irreversible, using glaucoma medications will not improve vision or restore visual field loss. Glaucoma is treated by lowering the intraocular pressure (IOP). The goal (or “target”) IOP after treatment depends on the individual eye and the status of optic nerve damage. Some patients may have nerve damage with IOP in the 20s (mm Hg) while others have worsening in the teens. Treatment should be tailored toward stabilization of an individual’s optic nerve and visual field. Most medical treatments for glaucoma involve eye drops (topical medications). Oral medications are also occasionally used to lower IOP.
Section 7-A: Aqueous Production and Outflow
Changes in IOP are made by regulating aqueous fluid (“humor”) production or drainage. Aqueous fluid is made in the ciliary body and travels between the iris and lens, and through the pupil. It then enters the anterior chamber and drains in the drainage angle (trabecular meshwork, see figure 7-1). Medications either decrease the production of aqueous fluid (called “aqueous suppressants”) or facilitate its outflow from the eye (referred to as “outflow drugs”) to lower the IOP. There are two main outflow passages from the eye: the conventional outflow and uveoscleral outflow. The conventional outflow provides a majority of the aqueous drainage through the trabecular meshwork. The non-conventional outflow, or uveoscleral outflow, provides the remaining aqueous outflow through the ciliary body face and iris. Outflow drugs may affect one or the other outflow pathways to lower IOP.
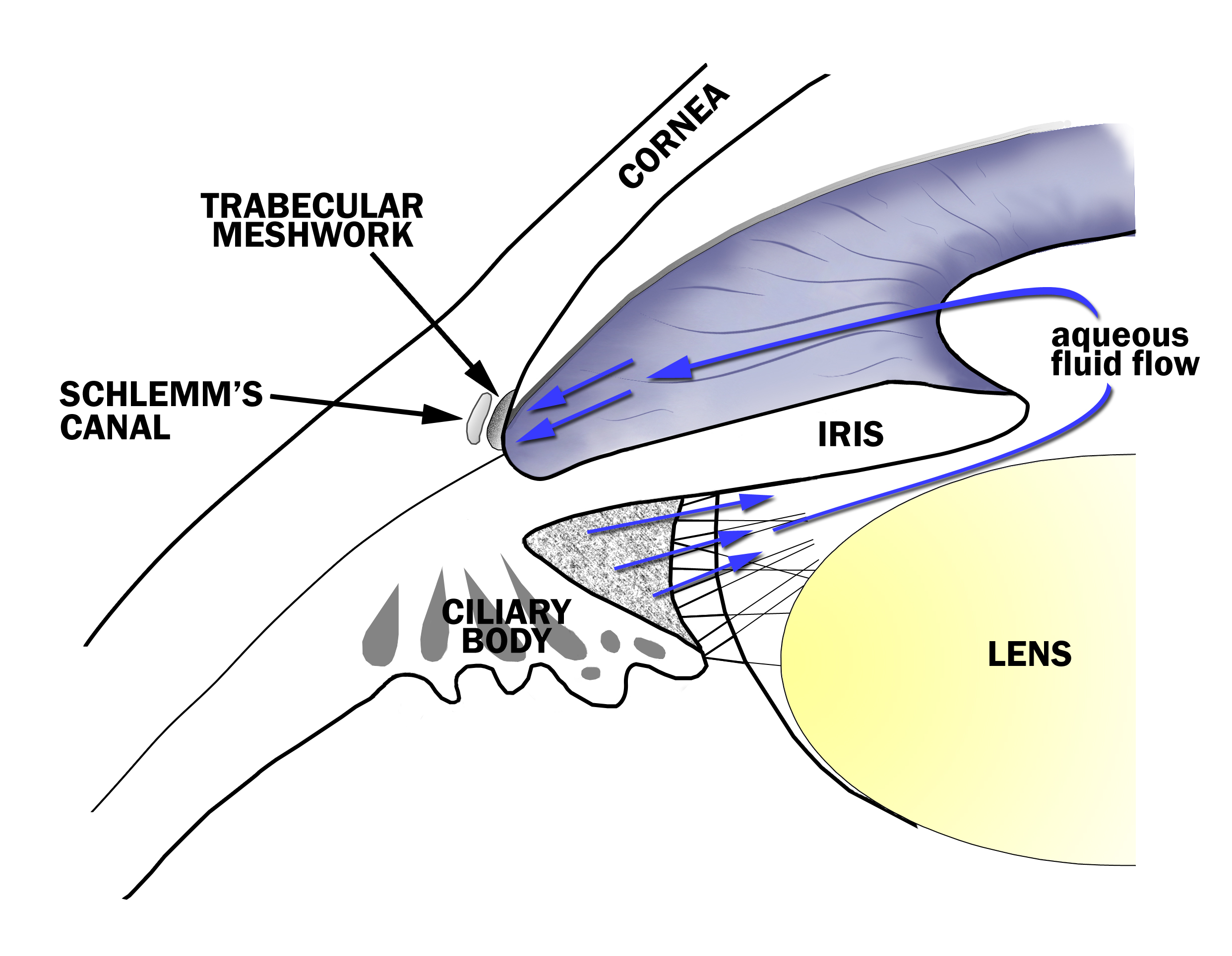
Figure 7-1. Aqueous Fluid Flow in the Eye. The aqueous fluid that fills the eye is produced by the ciliary body and flows between the iris and lens, through the pupil and to the drainage angle at the junction of the iris and the cornea. Aqueous fluid exits the eye through a tissue called the trabecular meshwork in the drainage angle.
Section 7-B: Topical Glaucoma Medications
There are multiple classes of topical medications for the treatment of glaucoma. Most either lower aqueous production (“aqueous suppressants”) or increase its outflow (“outflow drugs”) (Table 7-1). Some medications may have effect on both. Each class of eye drops can be identified by its cap color (Table 7-2).
| Decrease aqueous fluid production | Increase aqueous fluid outflow |
|---|---|
| Beta-blockers | Cholinergics |
| Alpha-adrenergics | Prostaglandin analogs |
| Carbonic anhydrase inhibitors |
The choice among the various treatments depends upon a patient’s health and ocular history. Although these medications are administered topically on the eye, they can have both ocular and systemic side effects. As with any medication, allergies or intolerances may develop with continued use. Common signs and symptoms of allergy are redness, itching, burning, and swelling. If any of these develop, the treating doctor should be contacted and may require discontinuing the medication.
| Class of Medication | Eye Drop Cap Color |
|---|---|
| Prostaglandins | Clear/teal |
| Beta-blockers | Yellow, blue |
| Alpha-adrenergics | Purple |
| Carbonic anhydrase inhibitors | Orange |
| Cholinergics | Green |
7C: Glaucoma Medication Classes
Section 7-C-1: Prostaglandin Analogs
Prostaglandins are chemicals made within the tissues of the body. Receptors exist for each of the various prostaglandins and have different physiologic effects on the body. When activated by prostaglandin PGF2alpha, the nonconventional outflow (uveoscleral outflow) is improved with resulting decrease in IOP.
Latanoprost (Xalatan, Figure 7-2 A) was the first prostaglandin developed for the treatment of glaucoma and has been shown to effectively lower IOP with once daily dosing at bedtime. It is suggested that unopen bottles of latanoprost be refrigerated, and once open, can be stored at room temperature. Similar agents, such as travoprost (Travatan, Figure 7-2 B) and bimatoprost (Lumigan, Figure 7-2 C), have been shown to lower IOP with once daily dosing as well. Unoprostone (Rescula) is another prostaglandin analog; it has the disadvantage of being dosed twice daily.

A. Latanoprost 0.005% (Xalatan, Pfizer Inc., New York, NY)

B. Travoprost 0.004% (Travatan, Alcon Inc., Fort Worth, TX)
C. Bimatoprost 0.03% (Lumigan, Allergan Inc., Irvine, CA)
Prostaglandins are usually the first line agents in the treatment of glaucoma. They are well-tolerated and lack significant systemic side effects. They have the added benefit of once daily dosing.
Ocular side effects include redness, irritation, corneal toxicity in those with a history of herpes simplex keratitis, inflammation, increased pigmentation of periocular skin, iris darkening (especially in hazel/green eye color), and increased length and number of eyelashes. As noted in Table 7-3, the side effects of prostaglandins are primarily ocular. No significant systemic side effects from prostaglandins give it an added advantage over other glaucoma medications. Another added benefit is its once daily dosing. In addition, the ocular side effects are often well-tolerated. The redness and irritation that occur initially tend to improve with time.
| Ocular side effects of prostaglandins |
|---|
| Redness |
| Irritation |
| Exacerbation of herpes simplex keratitis |
| Inflammation |
| Change of periocular skin/iris color |
| Increased length and number of lashes |
Section 7-C-2: Beta-blockers
Beta-adrenergic blockers (beta-blockers) and alpha-adrenergic agonists (see Section 7-C-3) work to lower IOP by affecting the autonomic (or sympathetic) nervous system. This system exists throughout the body interacting with alpha or beta receptors on cell membranes. Blockers inhibit the action at the receptor while agonists stimulate it. The effects are numerous but in the eye, the main effects are on aqueous production and pupillary constriction/dilation. The two main adrenergic receptors are the alpha and beta receptors. Within each class of receptor, there are subclasses having specific effects. Some medications are selective, meaning that they affect a specific subclass only, while others are nonselective, and they affect the entire class as a whole.
Beta blockers lower IOP by decreasing the production of aqueous. The most commonly used beta-blocker eye drop is timolol (brand name: Timoptic, Timoptic XE, Betimol, Istalol). The usual concentrations are 0.25% and 0.5%. It is very well tolerated and used either once or twice daily. It comes in both liquid and gel forms. The gel form typically is dosed once daily but has the disadvantage of causing transient blurring of vision. Timolol is easily identified by its cap color of yellow.
Beta-blocker eye drops (brand names in parenthesis) approved for glaucoma treatment include timolol (Timoptic, Betimol, Istalol), levobunolol (Betagan), carteolol (Ocupress), metopranolol (OptiPranolol) and betaxolol (Betoptic).
Timolol is the most commonly used nonselective beta-blocker and blocks both the beta1 and beta2 receptors. Stimulation of the beta1 receptor increases cardiac contractility, and the beta2 receptor affects bronchodilation. A nonselective blocker inhibits cardiac contractility and bronchodilation. These are, therefore, contraindicated in patients with asthma, emphysema, chronic obstructive pulmonary disease (COPD), bradycardia (low pulse rate), and congestive heart failure. Topical beta blockers have been widely used for glaucoma treatment since early 1980’s due to their efficacy and cost effectiveness. Studies have noted systemic side effects from absorption into the bloodstream from topical application of timolol. Topical beta-blockers may lose their effectiveness over time (tachyphylaxis) in some patients. If this occurs, another medication may be used to control the IOP. Other nonselective beta-blockers include levobunolol (Betagan), metipranolol (OptiPranolol), and carteolol (Ocupress). Other beta-blockers are selective for a specific beta receptor. Betaxolol (Betoptic) is a selective beta1 receptor antagonist. Its mechanism of action is similar to timolol, but since it is a selective beta1 blocker, it is better tolerated in patients with pulmonary disease than timolol. It could still have a slight beta2 blocking effect and thus, patients who develop shortness of breath should discontinue this medication.
Oral beta-blockers are often used for cardiovascular reasons. Concurrent use of oral beta-blockers may reduce the efficacy of the topical beta blocker. The main ocular side effects include redness, burning, scarring, decreased corneal sensation, decreased ocular blood flow, and inflammation. Although there are numerous reported ocular side effects, beta-blockers are usually very well tolerated. The main systemic side effects include decreased heart rate (bradycardia) and cardiac contractility, irregular heart rhythms (arrhythmias), worsening of congestive heart failure, bronchospasm, difficulty breathing, masking of low blood sugar in diabetics, and depression (Table 7-4). If any of the side effects occur with administration of beta-blocker eye drops, the medication should be discontinued by the prescribing doctor.
| Ocular | Systemic |
|---|---|
| Redness | Bradycardia (low pulse rate) |
| Burning | Arrhythmias (Irregular pulse) |
| Scarring | Worsening of congestive heart failure |
| Decreased corneal sensation | Bronchospasm |
| Decrease ocular blood flow | Masking of hypoglycemic (low glucose level) symptoms |
| Depression |
Section 7-C-3: Alpha-Adrenergic Agonists
Stimulation of the alpha1-adrenergic receptor produces dilation of the pupil, and the alpha2 receptor decreases aqueous production at the ciliary body. They inhibit the production of aqueous, but they can also facilitate the outflow of aqueous from the eye. Both mechanisms act to lower the IOP. Alpha2-specific adrenergic agents include brimonidine (Alphagan, Alphagan-P) and apraclonidine (Iopidine). Older, non-specific adrenergic agents include epinephrine and dipivefrin (Propine). Dipivefrin is a “pro-drug” which is converted in the eye into epinephrine, but the non-specific adrenergic agonists such as dipivefrin are rarely used any more.
Topical use of epinephrine and dipivefrin was also associated with significant side effects. These include high blood pressure, rapid heart rate (tachycardia), irregular heart beat (arrhythmias), burning, redness, conjunctival pigmentation, pupillary dilation, and swelling in the macula (cystoid macular edema) in patients who had previous cataract surgery without implantation of an intraocular lens implant. The more specific alpha2-adrenergic agonists, such as brimonidine or apraclonidine, are better tolerated compared to the older nonspecific adrenergic agents. Apraclonidine (Iopidine) was initially reported to be effective for the treatment of short-term IOP elevations after eye laser procedures. Brimonidine is the second generation of alpha2-agonists and has largely replaced apraclonidine. A common side effect of Alphagan is redness and ocular allergy. Alphagan-P contains a different preservative (Purite) which helps reduce the preservative-related allergy. Brimonidine is usually well tolerated and effective in lowering IOP. Common ocular side effects include allergic symptoms of redness, itching, and irritation. Pupillary dilation and upper lid retraction are also seen. Systemic side effects are uncommon but can include sedation, headache, and fatigue. Brimonidine should not be used in children under 2 and should be used with caution under the age of 5 due to respiratory and central nervous system depression (Table 7-5). It is contraindicated in patients taking monoamine oxidase inhibitors due to drug interactions.
| Ocular | Systemic |
|---|---|
| Redness | Hypotension (low blood pressure) |
| Itching | Respiratory depression (especially in infants), Central nervous system depression (especially in infants). |
| Irritation | Central nervous system depression (especially in infants) |
| Pupil dilation | Sedation |
| Lid retraction | Fatigue |
| Depression |
Section 7-C-4: Carbonic Anhydrase Inhibitors (CAIs)
The topical CAIs include dorzolamide (Trusopt) and brinzolamide (Azopt). Both are much better tolerated than the systemic CAIs due to reduced systemic side effects. Both are dosed either two or three times daily. In addition, dorzolamide also comes in a combined form with timolol (Cosopt, Figure 7-3) for better compliance in administration. The CAIs inhibit the enzyme carbonic anhydrase, which in turn, reduces aqueous humor formation. Patients are typically placed on topical glaucoma medications prior to resorting to oral CAIs due to their systemic side effects which are often poorly tolerated. Topical CAIs are effective at lowering the IOP. They are well-tolerated and have limited systemic side effects. Ocular side effects include irritation, redness, bitter taste after administration, and corneal toxicity. Although much less frequent compared with systemic CAIs, they may produce a bitter taste and low blood cell counts (Table 7-6).

Figure 7-3. Combination beta-blocker and carbonic anhydrase inhibitor: timolol maleate/dorzolamide hydrochloride (Cosopt, Merck Co., Whitehouse Station, NJ)
| Ocular | Systemic |
|---|---|
| Stinging | Allergic symptoms |
| Irritation | Bitter taste with administration |
| Redness | Low blood counts |
| Corneal toxicity |
The CAIs are the only class of chronic glaucoma therapy which is administered systemically (orally). Acetazolamide (Diamox) and methazolamide (Neptazane, GlaucTabs) are the two oral CAIs which may be used for the treatment of glaucoma. Methazolamide is usually better tolerated since it has fewer side effects than acetazolamide. However, it may not be as effective as acetazolamide. Reported side effects include tingling of fingers/toes (paresthesias), urinary frequency, electrolyte imbalances, fatigue, anorexia, depression, low potassium levels (hypokalemia), poor taste, nausea, diarrhea, kidney stone formation (nephrolithiasis), and low blood cell counts. Systemic CAIs are contraindicated in those with an allergy to sulfa, since it is a sulfonamide drug, and in those with a history of renal disease, kidney stones, low potassium (especially in those taking thiazide diuretics), or abnormal blood counts. Oral CAIs may exacerbate these conditions, and should be avoided or used with caution (Table 7-7).
| Systemic side effects of systemic Carbonic Anhydrase Inhibitors |
|---|
| Tingling of fingers/toes |
| Urinary frequency |
| Electrolyte imbalances |
| Fatigue |
| Depression |
| Low potassium levels |
| Metallic taste |
| Nausea |
| Diarrhea |
| Kidney stone formation |
| Abnormal blood counts |
Section 7-C-5: Cholinergic Agents
The cholinergic agonists act at a receptor called the muscarinic receptor. Once the receptor is activated, the outflow of aqueous through trabecular meshwork is increased. The pupil also constricts (miosis). Agents within this class can act directly at the receptor or indirectly by inhibiting the breakdown of the neurotransmitter acetylcholine by the enzyme acetylcholinersterase. The topical medications within this class include pilocarpine, echothiophate iodide, and carbachol. With the number of better tolerated and more easily administered glaucoma drops on the market, these agents are no longer used commonly.
Direct-Acting Cholinergic - Pilocarpine
Pilocarpine is a direct-acting cholinergic agonist. It opens the drainage angle and increases the trabecular meshwork aqueous outflow. There are different percentages of pilocarpine, and it has been noted that darker eye colors may require higher dosages of the medication. Dosing is frequently four times daily which can be difficult for a patient to comply. Pilocarpine was used frequently in the past due to its efficacy with rare systemic side effects (Table 7-8).
| Ocular | Systemic |
|---|---|
| Ciliary muscle spasm (eye ache) | Headache |
| Pupillary constriction | Perspiration |
| Retinal detachment (rare) | Stimulation of salivary, lacrimal, gastrointestinal glands |
| Corneal toxicity | Increased bronchial secretions |
| Inflammation | Worsening of Alzheimer’s disease |
Indirect-Acting Cholinergics – Carbachol, Echothiophate Iodide
Carbachol is both a direct and indirect-acting agent which is used as a topical medication. Echothiophate iodide (Phospholine Iodide) is an indirect-acting agent which inhibits acetylcholinesterase. The side effects of carbachol are similar to pilocarpine. Both carbachol and echothiophate iodide are not used commonly due to newer and more easily tolerated agents on the market. Although they are effective in lowering IOP, they can have unwanted side effects which make newer agents more attractive. Occasionally patients will continue on these medications if they were initially started on them with good success and tolerance. A potentially serious complication of echothiophate iodide is pseudocholinesterase depletion. Echothiophate iodide may also inhibit pseudocholinesterase which breaks down succinylcholine, a paralytic medication sometimes used for general anesthesia. There can be prolonged respiratory paralysis if used prior to general anesthesia. Other side effects include nausea, diarrhea, cataracts, iris cysts, and ocular inflammation (Table 7-9).
| Ocular | Systemic |
|---|---|
| Ciliary muscle constriction | Headache |
| Pupillary constriction | Respiratory depression |
| Retinal detachment | Nausea |
| Cataract formation | Diarrhea |
| Iris cysts | Abdominal cramping |
| Corneal toxicity | Malaise |
| Inflammation | Prolonged paralysis by succinylcholine during general anesthesia |
Section 7-D: Using Glaucoma Medications
Practice with instilling eyedrops improves the success of administering them correctly (Figure 7-4). The eye can normally hold 7 - 9 µl, and the average drop from an eye dropper or dropper tip is 39 µl. If half of the eye drop fails to stay in the eye, there is still enough retained in the eye to be absorbed. If multiple eye drops are being applied to the same eye, it is best to wait 10 minutes between eye drops to enhance drug absorption. Another way to improve absorption (and to decrease the systemic side effect) is to occlude the tear drainage from the eye by nasolacrimal occlusion (Figure 7-5). This is performed by pressing on the tear ducts near the inner lower corner of the eyes. Eyelid closure for several minutes after eye drop instillation has also been noted to be helpful.
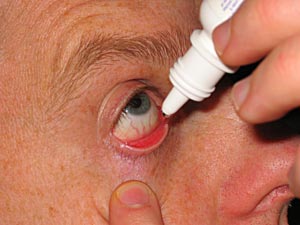
Figure 7-4. One glaucoma drop is placed on the eye while retracting the lower eyelid.
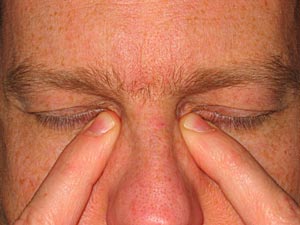
Figure 7-5. Simple eyelid closure or occlusion of the tear drainage system (punctual occlusion) helps improve ocular absorption while minimizing systemic absorption.
If maximum glaucoma medications fail to sufficiently lower the IOP, then further treatment is indicated with either laser or surgery. The surgical treatment options are based on the stage of glaucoma and anatomic characteristics of each individual.
Chapter 7. References
Allingham RR, et al., editors. Shields’ Textbook of Glaucoma, ed. 5. Philadelphia: Lippincott Williams & Wilkins, 2005. pp. 457-508.
Alward WLM. Glaucoma. St. Louis: Mosby, 2000. pp. 186-205.
Bartlett, JD, editor. “Agents for Glaucoma.” IN Ophthalmic Drug Facts. St. Louis: Facts and Comparisons, 2003. pp. 181-252.
Bartlett JD. Jaanus SD, Fiscella RG, Sharir M, “Ocular Hypotensive Drugs.” IN Bartlett JD, Jaanus SD, eds. Clinical Ocular Pharmacology, ed. 4. Boston: Butterworth-Heinemann, 2001. pp. 167-218.