
Section 10-A: Types of Pediatric Glaucoma
Pediatric glaucoma (also referred to as childhood glaucoma, infantile glaucoma or congenital glaucoma) is a relatively rare disease, as most patients with glaucoma are adults. However, pediatric glaucoma can lead to loss of vision and blindness in a young child and will profoundly affect the child’s life, if not diagnosed promptly and treated appropriately. Pediatric glaucoma can include a number of different diagnoses. Primary congenital glaucoma occurs in the first three years of life (usually within the first 6 months of life) without associated ocular or systemic abnormalities. Other pediatric glaucomas are associated with ocular and/or systemic abnormalities, or due (secondary) to another disease (Table 10-1). The remainder of this chapter describes the primary congenital glaucoma.
| Primary pediatric glaucoma associated with ocular abnormalities | Primary pediatric glaucoma associated with systemic abnormalities | Secondary pediatric glaucoma |
|---|---|---|
| Aniridia | Axenfeld-Rieger syndrome | Trauma |
| Axenfeld-Rieger syndrome | Congenital rubella | Intraocular tumors (e.g., retinoblastoma) |
| Congenital hereditary endothelial dystrophy (CHED) | Cutis marmorata telangiectasia congenita | Uveitis (ocular inflammation) |
| Congenital microcornea with myopia | Marfan syndrome | Lens-induced |
| Congenital ocular melanosis (Nevus of Ota) | Neurofibromatosis 1 | Aphakic (after cataract surgery without lens implant) |
| Peters anomaly | Oculocerebrorenal (Lowe) syndrome | Steroid-induced |
| Posterior polymorphous dystrophy (PPMD) | Stickler syndrome | Ocular infection |
| Sclerocornea | Sturge-Weber syndrome | Angle-closure (e.g., retinopathy of prematurity) |
The primary congenital glaucoma is thought to be an autosomal recessive disease and can be associated with positive family history. However, in many cases of primary congenital glaucoma, there is no obvious family history. It occurs approximately one in 30,000 live births, and the risk for congenital glaucoma is increased if there is a pre-existing family history. Molecular genetic studies have identified CYP1B1 gene as a cause for congenital glaucoma in patients with positive family history (see Chapter 11 for more).
Section 10-B: Diagnosis of congenital glaucoma
Congenital glaucoma occurs in infants up to 3 years of age. Most commonly, patients are diagnosed between 3-6 months of age. In the US, boys are slightly more commonly affected than girls. Approximately 70% of congenital glaucoma patients have both eyes affected, while the remaining 30% have only one eye affected. There are 3 common symptoms associated with congenital glaucoma ( Table 10-2). These include tearing (epiphora. Figure 10-1), light sensitivity (photophobia, Figure 10-2), and spasm and closure of the eyelids (blepharospasm, Figure 10-2) due to the patient’s sensitivity to light.
.jpg)
Figure 10-1. Light sensitivity and tearing in a teenage patient with a long history of congenital glaucoma. He also has misalignment of the eyes ( strabismus).
.jpg)
Figure 10-2. Spasm and closure of the eyelids (blepharospasm) and light sensitivity (photophobia) of a patient with congenital glaucoma.
| Common symptoms of congenital glaucoma | Common signs of congenital glaucoma |
|---|---|
| Tearing ( epiphora) | Elevated intraocular pressure (IOP) |
| Light sensitivity (photophobia) | Enlarged cornea |
| Spasm and closure of eyelids (blepharospasm) | Cloudy, hazy cornea (corneal edema) |
| Tears in Descemet’s membrane in cornea (Haab’s striae) | |
| Enlarged length of the eye (buphthalmos) | |
| Optic nerve damage (cupping) |
On eye examination, there are certain features that are commonly associated with congenital glaucoma (Table 10-2). Patients often avoid bright lights because they are very sensitive to light. This is from the cloudy, hazy cornea that disperses the light and causes glare. Instead of a normally clear cornea, these patients develop a thick, cloudy, and hazy cornea (frosted glass appearance) from elevated intraocular pressure (IOP). In severe cases, the patient’s pupil may not even be visible due to the cloudy cornea. Over time, the elevated IOP can cause “stretch marks” in the cornea from excessive stretching (Haab’s striae, Figure 10-3), and also enlarge the size of the eye itself (buphthalmos, Figure 10-4).
.jpg)
Figure 10-3. Stretch marks in the cornea (Haab’s striae) from the high intraocular pressure in a patient with congenital glaucoma.
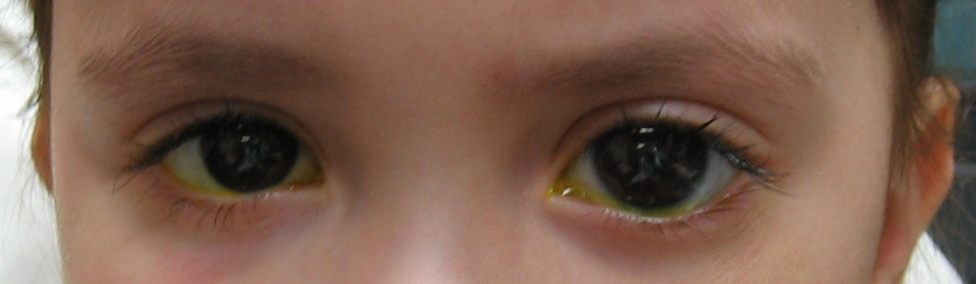
Figure 10-4. The left eye of this congenital glaucoma patient is noticeably larger than the right eye. The patient has buphthalmos of the left eye
Abnormal enlargement of one or both eyes in an infant is an important sign of congenital glaucoma and should not be ignored. Eventually, as in adult glaucoma, the optic nerve will become damaged (cupping, see Chapter 1). However, unlike adult glaucoma, the optic nerve damage in congenital glaucoma is considered reversible in the early stages if the glaucoma is treated promptly and effectively.
Section 10-C: Treatment of congenital glaucoma
Unlike adult glaucoma, the initial treatment for congenital glaucoma is often surgical. A “drainage angle surgery” is often recommended for congenital glaucoma. The most common surgical procedures for congenital glaucoma are goniotomy and trabeculotomy. While they are considered to have similar rates of success (80-90%), some surgeons prefer one technique over the other. One advantage of trabeculotomy over goniotomy is that a clear cornea is not necessary to perform the procedure, while a reasonably clear cornea is necessary for goniotomy. The goniotomy surgery involves entering the anterior chamber with a sharp goniotomy knife and making an opening incision through the abnormally developed trabecular meshwork to allow greater outflow of the aqueous fluid and thereby, lower the IOP (Figure 10-5). Often 120 degrees (out of 360 degrees total) of the trabecular meshwork can be treated with goniotomy in a single setting. Trabeculotomy surgery involves making an external incision and identifying the Schlemm’s canal from the outside, inserting a fine instrument into the Schlemm’s canal, and breaking through the trabecular meshwork to increase the aqueous outflow (Figure 10-6). Typically, 120-140 degrees of trabecular meshwork can be treated by trabeculotomy in a single surgery. If one surgical technique is unsuccessful in decreasing the IOP, the other technique can be utilized in a fresh area of the trabecular meshwork (the area not previously operated upon) to increase the success of the surgery. Even after initial control of the intraocular pressure is established with surgery, a periodic monitoring is necessary to ensure the IOP doesn’t increase again and the glaucoma go out of control.
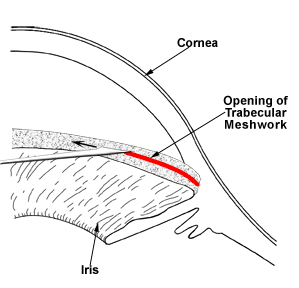
Figure 10-5. Goniotomy. A fine surgical knife is used to open the drainage angle (trabecular meshwork) in order to lower the intraocular pressure.
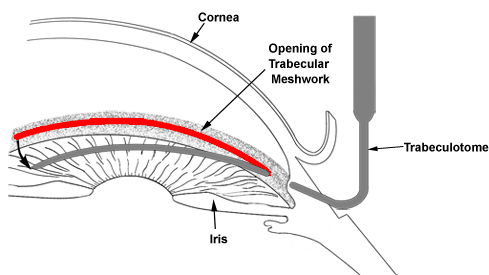
Figure 10-6. Trabeculotomy. A trabeculotome instrument is used to open the drainage angle (trabecular meshwork) in order to lower the intraocular pressure.
Medications can be used as an adjunct therapy either before or after the surgical treatment. Medications may be utilized temporarily after the diagnosis until surgery can be performed. If the initial surgery fails to completely control the IOP, topical medications can be used to bring the glaucoma under control. The systemic side effects of topical medications are greater in infants than in adults because of the smaller body mass. Because of potential systemic side effects, the first line of medications that are commonly employed is the topical carbonic anhydrase inhibitors (CAI, see Chapter 7). After the CAI, the next choices are topical prostaglandin analogs or beta-blockers (Chapter 7). The prostaglandin analogs appear to be safe in children; however, there are no long-term data on the safety of these medications in children. Topical beta-blockers should be used with caution in children because of the well-known systemic side effects (Chapter 7). Finally, topical alpha-2 agonist (brimonidine, Chapter 7) should be AVOIDED in infants because it’s been associated with severe respiratory depression (breathing difficulty).
Section 10-D: Importance of team approach in pediatric glaucoma
It takes a whole team of physicians, nurses, and family to provide an optimal treatment for patients with pediatric glaucoma. For example, a glaucoma specialist may provide care for pediatric glaucoma, while a pediatric ophthalmologist may simultaneously treat a “lazy eye” (amblyopia). Lazy eye is a condition in which the visual part of the child’s brain does not develop properly due to abnormalities of the eye (for example, glaucoma) or eye alignment (strabismus). Pediatric glaucoma patients are at a high risk for development of amblyopia, unless they are closely monitored and treated. If amblyopia is detected, he/she needs to be promptly treated because the amblyopia becomes irreversible and not amenable to treatment after the age of approximately 10 years.
It is critical to have a total commitment of the family as well. The family is asked to bring the patient to multiple doctors over many months or years for the treatment of glaucoma. The child may undergo multiple sedations or anesthesia, just to check the intraocular pressure and perform adequate ocular examination. The family is often asked to administer multiple eye drops every day as part of the glaucoma treatment. All of these activities can add a significant amount of stress to the family as well as the patient. On the other hand, when everyone works together to provide an optimal treatment, there is a good chance that the child with glaucoma can grow up with good eyesight.
Chapter 10. References
Allingham RR, Damji K, Freedman S, Moroi S, Shafranov G. Ch 13: Congenital Glaucomas. In: Shield’s Textbook of Glaucoma. 5th Ed. Lippincott Williams and Wilkins, Philadelphia, p235-251, 2005.
Alward, WLM. Ch 9: Glaucomas of Infancy and Childhood. In Glaucoma: The Requisites in Ophthalmology, Mosby, St. Louis, p111-127, 2000.