
Section 2-A: Introduction
Glaucoma represents a significant public health problem. Glaucoma affects more than 67 million people in the world, and approximately 10% of them are estimated to be blind from glaucoma. It is the leading cause of irreversible blindness in the world. In the United States, glaucoma affects more than 2.2 million people, and is the second leading cause of blindness in adults over the age of 40 (the first is macular degeneration).
Glaucoma is not a single disease. There are different types of glaucoma. Glaucoma can be broadly divided into two categories depending on whether or not the drainage angle (called trabecular meshwork) in the front of the eye (called anterior chamber) is open. The angle is formed by the juncture of the cornea and iris. The trabecular meshwork is where the eye fluid (called aqueous humor) normally drains out of the eye. If the drainage angle is open on examination, the patient is said to have “open-angle” glaucoma. If the drainage angle is closed on examination, the patient is said to have “angle closure” glaucoma. Refer to the next chapter (Chapter 3) for detailed description of the eye anatomy, as it relates to glaucoma. Epidemiology (study of disease in populations) of the open-angle glaucoma is sufficiently different from the angle closure glaucoma that each warrants a separate discussion.
Section 2-B: Epidemiology of Primary Open-Angle Glaucoma
The word “primary” of primary open-angle glaucoma indicates that no specific cause for the disease has been found to date. Primary open-angle glaucoma (POAG) is associated with optic nerve damage with resultant visual loss. However, POAG may or may not be associated with elevated intraocular pressure (IOP). The normal IOP is between 10 and 21 (in millimeters of Mercury or mm Hg). Classically, POAG occurs in a patient with elevated IOP (greater than 21). However, significant portion of POAG patients do not have elevated IOP; this subgroup of patients is often referred to as having “normal tension,” “normal pressure,” or “low tension” glaucoma.
It is estimated that normal pressure glaucoma makes up anywhere from 40-75% of all primary open angle glaucoma. And POAG makes up 85-90% of all glaucomas in the Western world. Prevalence of primary open angle glaucoma has been extensively studied by a number of well-designed clinical studies in different populations. In white populations, primary open angle glaucoma is present in 0.3 to 4.0% of the older population (Table 2-1). In Asian populations, POAG is present in 0.5 to 2.6% of the older population. In the Hispanic population in the United States, POAG is present in 2.0%; however, the number of studies on Hispanic populations is limited. In black populations, the prevalence of POAG is higher and ranges from 2.9 to 8.8% of the older population. It is clear that the black population is at a higher risk of developing POAG than in other populations.
| Race / Location | Prevalence of Primary Open-Angle Glaucoma in older age population (generally over age 40) |
|---|---|
| White populations (US, Europe, Iceland, Australia) | 0.3 – 4.0 % |
| Asian populations (Japan, Mongolia, Singapore, India) | 0.5 – 2.6 % |
| Hispanic population (US) | 2.0 % |
| Black populations (US, Caribbean, Africa) | 2.9 – 8.8 % |
In general, the risk of developing glaucoma is very small in general population (see Table 2-1). However, there are several risk factors that are associated with the development of POAG. As seen in Table 2-1, the black race is one such risk factor. Other risk factors include older age, positive family history of glaucoma, and elevated intraocular pressure. A black person has 4 times the risk of developing glaucoma than a white person. The older you are, the more likely you will develop glaucoma. If your first-degree relative (parent or sibling) has glaucoma, your chance of developing glaucoma increases by 2 to 4 fold. If your intraocular pressure is over 30 (mm Hg), the chance of developing glaucoma is 40 times greater than if the IOP is under 15. Thin cornea, which can lead to IOP measurement error (under-estimation), is also associated with increased risk of glaucoma. In other words if you have thin cornea, your true IOP is higher than what is measured, and you are at a higher risk of developing glaucoma. Additional minor risk factors for glaucoma include myopia (near sightedness), low diastolic blood pressure, and diabetes among others. Normal pressure glaucoma has been associated with migraine headache and Raynaud’s phenomenon (“cold and numb fingers” due to poor peripheral blood circulation).
It is uncommon, but still possible, for patients to become blind from primary open-angle glaucoma. Several studies suggest the risk of becoming blind in one eye for a glaucoma patient can range 15 to 54% over 15-22 year period. The risk of becoming blind in both eyes for a glaucoma patient can range from 4 to 22% over the same period. The risk factors for developing blindness from glaucoma include advanced stage of glaucoma at diagnosis, younger age at diagnosis, poor IOP control, poor compliance with medications, and inadequate treatment and follow-up care. Some of the blindness data are old (from before 1980); with the improvement in treatment, it is generally believed that the rate of blindness from glaucoma in the 21st century is lower than the figures shown above.
How do we detect glaucoma early so that we can prevent blindness from open-angle glaucoma? There has not been a simple answer. Population screening of intraocular pressure to detect glaucoma has not been very successful, because significant percentage of patients with glaucoma have normal IOP. On the other hand, examining everyone by an ophthalmologist would be prohibitively costly and time-consuming. The American Academy of Ophthalmology recommends a complete eye examination (that includes glaucoma screening) every 2-4 years between ages 40-64, then every 1-2 years after age 65 (Table 2-2). If you have risk factors for glaucoma, additional examinations are recommended.
| Age group | No glaucoma risk factors | With glaucoma risk factors |
|---|---|---|
| 20-29 | At least once during interval | Every 3-5 years |
| 30-39 | At least twice during interval | Every 2-4 years |
| 40-64 | Every 2-4 years | Every 2-4 years |
| 65+ | Every 1-2 years | Every 1-2 years |
Section 2-C: Epidemiology of Primary Angle Closure Glaucoma
Primary angle-closure glaucoma (PACG) is usually associated with elevated intraocular pressure, optic nerve damage, and resultant visual loss. The aqueous fluid drainage angle becomes progressively narrower (usually with age) and eventually, the intraocular pressure increases as a result of decrease in aqueous fluid drainage.
Epidemiology of PACG is less studied than that of open angle glaucoma. However, it is no less important. In fact, PACG may account for 64% of all glaucomas in Mongolia, and 50% of all glaucomas worldwide. In the white, Hispanic, and black populations, angle-closure glaucoma is present in 0.1 - 0.6% of the older population (Table 2-3). However in Asian populations, angle-closure glaucoma is present in 0.3% (Japan) to 2.7% (Alaska) of the older population.
| Race / Location | Prevalence of Angle-Closure Glaucoma in older age population (generally over age 40) |
|---|---|
| White populations (Europe, Australia) | 0.1 – 0.6 % |
| Asian populations (Alaska, Japan, Mongolia, Singapore, India) | 0.3 – 2.7 % |
| Hispanic population (US) | 0.1 % |
| Black populations ( Africa) | 0.5 – 0.6 % |
Blindness can occur in angle closure glaucoma as well. In fact, the rate of blindness from angle closure glaucoma may be even higher than that of open angle glaucoma. Blindness in one eye occurs in 10 –50% of Inuit and Chinese patients with angle closure glaucoma. In East Africa, blindness in both eyes occurs in 21% of angle-closure glaucoma patients.
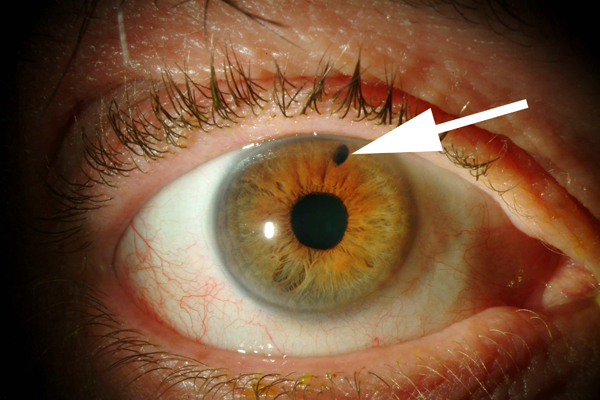
Figure 2-1. Photograph of an eye that has received laser peripheral iridotomy (LPI) to treat acute angle-closure glaucoma. LPI creates a small hole in the iris (arrow) that is visible with an eye examination.
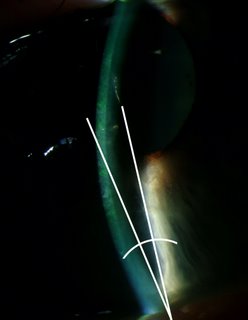
Figure 2-2. Slit lamp picture of an eye in acute angle closure glaucoma. Notice the drainage angle represented here by 2 intersecting lines is very narrow. The angle is also called “irido-corneal” angle because it is formed by the iris and cornea.
Because acute angle-closure glaucoma can be prevented with a laser surgery (see Figure 2-1, chapter 9 will cover this in more detail), there is a great interest in population screening for early detection of angle closure glaucoma. In reality however, screening for angle-closure glaucoma has been difficult. There are several methods for detection of narrow (or closed) drainage angle (Figure 2-2). Some are simple (for example, oblique flash light test), while others require special equipment (for example, Van Herick’s test utilizing the slit lamp instrument that most ophthalmologist use for examining the eye). Unfortunately, none of these methods meet all the criteria for effective mass screening, which requires quick, easy administration by minimally trained personnel at low cost. Studies are under way to look for most effective methods to screen for and prevent angle-closure glaucoma in high-risk Asian population. The population screening and preventive treatment of angle closure glaucoma is particularly important in Asian countries where the prevalence of angle closure glaucoma is relatively high. Such screening and preventive treatment of angle closure glaucoma can have major impact on public health in those populations.
Chapter 2. References
Allingham RR, Damji K, Freedman S, Moroi S, Shafranov G. Clinical Epidemiology of Glaucoma. In: Shield’s Textbook of Glaucoma. 5th Ed. Philadelphia: Lippincott Williams and Wilkins; 2005; p170-190.
Kwon YH, Caprioli J: Primary Open Angle Glaucoma. In: Duane’s Clinical Ophthalmology, Tasman W, Jaeger EA, eds.; Philadelphia: J.B. Lippincott Co.; 1999;, Chapter 52:1-30.
Kwon YH, Kim C, Zimmerman MB, Alward WLM, Hayreh SS. Rate of visual field loss and long-term visual outcome in primary open angle glaucoma. Am J Ophthalmol. 2001; 132(1): 47-56.