
Heredity plays an important role in most forms of glaucoma. In some types of glaucoma, such as in juvenile open angle glaucoma, disease clearly runs in families. However, the contribution of genetic factors to other types of glaucoma, such as primary open angle glaucoma, may be less obvious. Many genetic factors that are involved in the development of glaucoma are discussed in more detail below.
Section 11-A: The Genetic Basis of Glaucoma
Several lines of evidence indicate that glaucoma has a genetic basis, that is, glaucoma is caused in part by defects in specific genes (Table 11-1). First, although many cases of glaucoma occur with no family history of disease, glaucoma appears to be clearly heritable in some families. A number of large families have been reported in which glaucoma is inherited as a simple Mendelian trait (usually with autosomal dominant inheritance). Studies of the epidemiology of glaucoma also support the notion that there is a significant genetic component. Relatives of individuals affected with glaucoma have a much higher risk of developing glaucoma when compared to the general public. Additionally, many of the individual signs of glaucoma are heritable themselves, including cup to disc ratio and IOP. When these key features of glaucoma are examined individually, they appear to run in families. Lastly, the frequency of glaucoma varies greatly between different ethnic and racial groups. For example, the prevalence of glaucoma in African Americans is significantly higher than that of whites, which suggests that African Americans have a higher risk of developing glaucoma due to a heritable factor that is more prevalent in this racial group.
| Evidence that glaucoma is caused at least in part by genes. |
|---|
| Families that show clear inheritance of glaucoma |
| Relatives of glaucoma patients have a higher rate of developing glaucoma themselves |
| Features of glaucoma (large cup to disc ratio and high intraocular pressure) are heritable |
| Glaucoma is more common in some ethnic and racial groups than others |
Section 11-B. Research Methods for Studying Glaucoma Genetics
Two general approaches to study the genetics of glaucoma are candidate gene screening and positional cloning. The advantages of each of these types of investigations are discussed below.
Section 11-B(1). Candidate Gene Approach to Glaucoma
The core features of the candidate gene approach are:
1) making a list of candidate genes that might cause glaucoma if their normal function was altered and
2) testing a large group of unrelated glaucoma patients for defects in these candidate genes.
Identifying candidate genes.
Several types of genes are suspected of having a role in the development of glaucoma. Some of the best candidate genes have functions that suggest they may be important in glaucoma such as 1) genes that are active in the drainage angle where fluid leaves the eye;
2) genes that are active in the ciliary body where fluid enters the eye;
3) genes with functions that suggest they regulate the intraocular pressure; and
4) genes that may be important in maintaining the health of the optic nerve.
Testing candidate genes.
Candidate genes are evaluated for a possible role in causing glaucoma by testing the DNA of large numbers of unrelated glaucoma patients for disease-causing defects. Candidate gene screening is a useful research approach to discover disease-causing genes. This research is dependent on the enrollment of hundreds of volunteer subjects that have glaucoma. By participating in candidate gene studies of glaucoma, patients may learn something about the reasons why they developed glaucoma as well as help with research efforts to study the disease.
Section 11-B(2). Positional Cloning Approach to Glaucoma (Linkage Analysis)
Linkage analysis is a method for identifying glaucoma-causing genes that is dependent solely on the availability of large families with several members that have glaucoma. DNA is collected from each member of these families and is tested to see which segments of the DNA are always passed down through the family along with glaucoma. Genes that cause glaucoma are located within these linked regions of DNA (Figure 11-1).
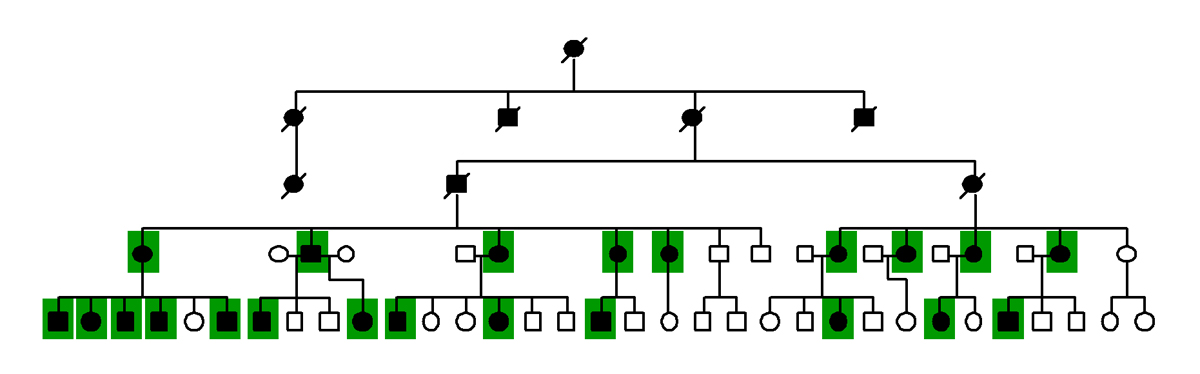
Figure 11-1. Linkage analysis of glaucoma. These diagrams represent the family tree of a family affected with glaucoma. The males in the family are represented by squares and the females are represented by circles. Deceased family members have a diagnonal line across the symbol. Family members affected with glaucoma are indicated by shading the pedigree symbols black. Most spouses were omitted from the diagram. In linkage analysis the inheritance of genetic markers (represented in the diagrams of a family tree with colored squares) is compared to inheritance of glaucoma (represented by darkly shaded pedigree symbols). The inheritance of a genetic marker on chromosome 1 is represented by the green boxes. Notice that the green boxes are always passed down through the family together with glaucoma. The gene causing glaucoma in this family is located near this genetic marker on chromosome 1.
Linkage analysis identifies where glaucoma genes are located within the genome. The next step is testing members of the family for disease-causing defects in the genes that are located with the linked regions.
Linkage analysis has some advantages that make it particularly well-suited for searching for glaucoma genes. This approach to finding disease-causing genes is possible even when little is known about the basic biological mechanisms of the disease being studied (such as glaucoma). This research is dependent on the identification and enrollment of large families with many members that are affected with glaucoma. Most of the known glaucoma genes were discovered with linkage analysis of large glaucoma families.
Section 11-C. The Genetics of Specific Types of Glaucoma
Several forms of glaucoma have been investigated in search of disease causing genes. The genes associated with juvenile open angle glaucoma, primary open angle glaucoma, primary congenital glaucoma, and many forms of secondary glaucoma have been identified (Table 11-2).
| Gene | Chromosomal location | Type of Glaucoma |
|---|---|---|
| Myocilin (MYOC) | Chromosome 1q24.3-q25.2 | JOAG or POAG |
| Optineurin (OPTN) | Chromosome 10p15-p14 | NTG |
| Cytochrome P450 1B1 (CYP1B1) | Chromosome 2p22-p21 | PCG |
| Paired homeodomain transcription factor 2 (PITX2) | Chromosome 4q25-q26 | Glaucoma associated with ARS |
| Forkhead Box C1 (FOXC1) | Chromosome 6p25 | Glaucoma associated with ASD and ARS |
| Paired box 6 (PAX6) | Chromosome 11p13 | Glaucoma associated with aniridia |
Section 11-C(1). Juvenile Open Angle Glaucoma
Juvenile open angle glaucoma (JOAG) is a rare form of glaucoma that accounts for approximately 1% of total glaucoma patients. The clinical features of JOAG are the same as those of more common forms of glaucoma (such as POAG). JOAG differs from POAG mainly in the severity of disease and age of onset. Patients with JOAG develop disease at a much earlier age than patients with POAG (between 3 and 40 years of age). JOAG patients also have very high intraocular pressures that frequently exceed 40 mm Hg in the absence of treatment.
In many cases, JOAG clearly runs in families as a dominant trait. Due to the early age of onset and the strong clinical signs of JOAG, several large pedigrees with many generations of affected family members have been recognized (Figure 11-2).
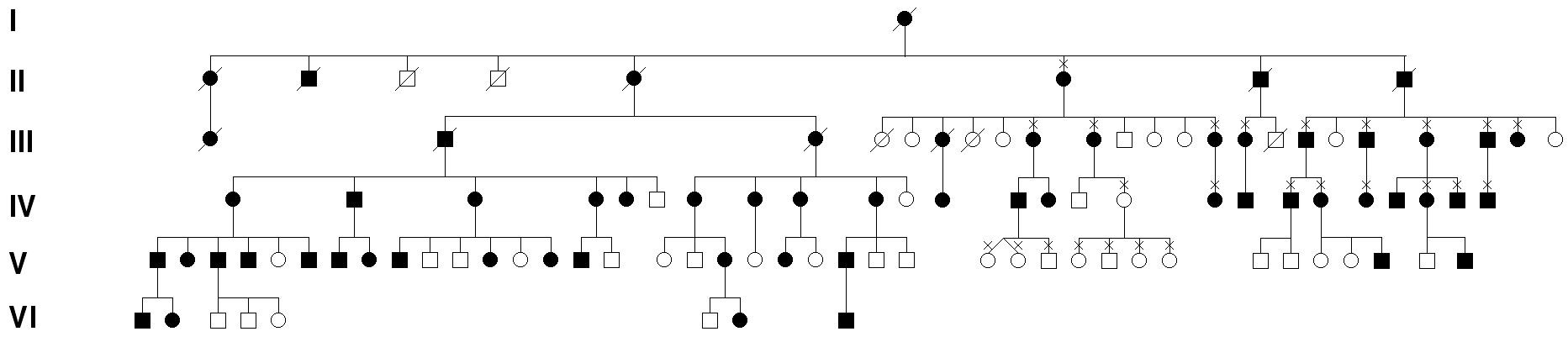
Figure 11-2. Juvenile open angle glaucoma (JOAG) pedigree. JOAG is an early-onset form of open angle glaucoma that is inherited as an autosomal dominant trait. Offspring of a parent with JOAG have up to a 50% chance of inheriting JOAG. This diagram shows the pattern of inheritance of glaucoma through an actual JOAG pedigree.
Genetic studies of large families (like the one shown in Figure 11.2) demonstrated that defects or mutations in the myocilin gene are a cause of JOAG. Most cases of JOAG that have a strong family history of disease are associated with defects in the myocilin gene (MYOC). Myocilin associated glaucoma is inherited as an autosomal dominant trait. That is, patients carrying a myocilin mutation that causes JOAG have a 50% chance of passing the gene (and high risk for glaucoma) to their children. Several specific defects or mutations in the myocilin gene that cause JOAG have been identified.
Some patients have the typical clinical features of JOAG but do not have a family history of disease. The myocilin gene has a less important role in these sporadic cases of JOAG.
The myocilin gene directs tissues of the eye to produce a protein that is released into the aqueous humor. The myocilin protein has an unknown function; however, glaucoma develops when its structure is altered by a mutation. Studies are underway to investigate role of the myocilin gene in healthy eyes and the process by which defects in this gene lead to glaucoma.
Section 11-C(2). Primary Open Angle Glaucoma
Primary open angle glaucoma (POAG) is the most common form of glaucoma in the United States. Like juvenile onset open angle glaucoma (JOAG), there is also a genetic basis to POAG. However, there are important differences between the genetics of POAG and JOAG. POAG runs in families, but the pattern of inheritance is more difficult to recognize. Due to the relatively late onset of disease in POAG (after the age of 40), most of the families with this condition only include one or two generations of affected family members that are alive. Parents of affected family members are often deceased and offspring of affected members are frequently too young to show signs of the disease. Consequently, families with inherited forms of POAG are generally small with only a few affected members and are difficult to distinguish from sporadic (non-familial) cases (Figure 11-3).
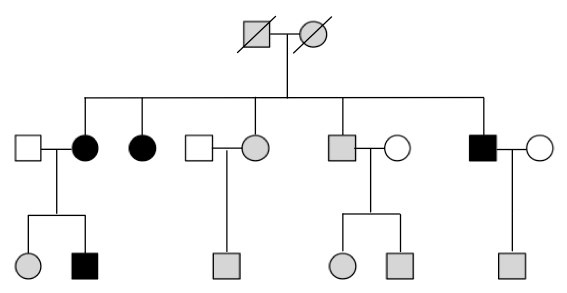
Figure 11-3. POAG pedigree. This diagram shows an example of the pattern of inheritance of glaucoma may occur in a POAG pedigree. The squares represent male family members, while the circles represent female family members. Darkly shaded symbols indicate an affected family member, while unshaded symbols indicate an unaffected family member. Grey symbols indicate a family member with unknown glaucoma status. Diagonal lines (through a square or circle) indicate that a particular family member is deceased. Notice that the founders of the family are deceased and it is unknown whether they were affected with POAG (indicated by grey boxes). Similarly, most of the grandchildren of the founders are younger than the age at which most people develop glaucoma. It is unknown whether these family members will later develop glaucoma so they are shaded grey to indicate their unknown glaucoma status. The majority of family members affected with POAG are in a single generation.
Although research has indicated that POAG is heritable, most of the genes that cause this disease have not yet been identified. It is likely that some cases of POAG will be due to defects in a single gene, while other cases will be due to the combined effects of mutations in several genes and other environmental factors.
The identification of disease-causing genes has been an active focus of research studies of POAG. The approximate position in the genome, or locus, of many genes that can cause POAG has been determined by linkage analysis (Table 11-3). Loci for genes that cause POAG are designated by the a code beginning with the letters “GLC1” and ending in a suffix letter for each new glaucoma loci in chronologic order of discovery. For example, GLC1A was the first open angle glaucoma locus to be discovered. The glaucoma genes in two of these loci have been discovered (myocilin at the GLC1A locus and optineurin at the GLC1E locus). Research to find the disease-causing genes at the other loci is ongoing.
| Chromosomal location | Locus Name | Known Gene | Glaucoma |
|---|---|---|---|
| Chromosome 1q | GLC1A | (MYOC) | JOAG, POAG |
| Chromosome 2q | GLC1B | - | POAG |
| Chromosome 3q | GLC1C | - | POAG |
| Chromosome 8q | GLC1D | - | POAG |
| Chromosome 10p | GLC1E | (OPTN) | NTG |
| Chromosome 7q | GLC1F | - | POAG |
| Chromosome 5q | GLC1G | - | POAG |
| Chromosome 2p | GLC1H | - | POAG |
| Chromosome 15q | GLC1I | - | POAG |
| Chromosome 9q | GLC1J | - | POAG |
| Chromosome 20p | GLC1K | - | POAG |
| Chromosome 2p | GLC3A | (CYP1B1) | PCG |
| Chromosome 1p | GLC3B | - | PCG |
At present, only one gene that causes POAG has been discovered (the myocilin gene at the GLC1A locus). As discussed above, one set of mutations in the myocilin gene are known to cause early onset glaucoma (JOAG). A different set of mutations in the same myocilin gene can cause POAG. In fact mutations in the myocilin gene have been shown to be responsible for approximately 3-4% of worldwide cases of POAG. Myocilin associated glaucoma is inherited as an autosomal dominant trait and offspring of affected parents have a 50% chance of inheriting an abnormal myocilin gene, which confers a high risk for developing glaucoma.
Myocilin defects have been identified in patients with POAG from different races and ethnicities including Caucasians from Midwestern America; Caucasians from Canada; Caucasians from Australia; African Americans from New York City; and Asians from Gifu, Japan. In all populations approximately 1 in 25 cases of POAG are due to abnormalities of the myocilin gene. One set of defects in the myocilin gene cause JOAG while a different set of defects cause POAG. In many cases, when a particular defect in the myocilin gene is detected, the severity of the associated glaucoma (age of onset and maximum intraocular pressure) may be accurately predicted.
Myocilin mutations account for approximately 1 in 25 cases of primary open-angle glaucoma. It is likely that many additional genes are involved in the development of glaucoma. The search for these genes is an area of ongoing research.
Section 11-C(3). Normal Tension Glaucoma
Most cases of normal tension glaucoma (NTG) are sporadic with no clear family history. However, rare cases of families with many affected members have been reported. In these families, NTG is inherited as an autosomal dominant trait. Genetic studies have shown that mutations in a gene known as optineurin (OPTN) are responsible for a significant fraction of familial NTG cases. A single optineurin mutation has been associated with glaucoma in several large NTG families. The role of the optineurin gene in sporadic cases of NTG has not been clearly defined.
More is known about the optineurin gene than the myocilin gene. The optineurin gene produces a protein that appears to have many functions. Some studies suggest that optineurin is involved in apoptosis, which is a process by which cells self-destruct or “commit suicide”. It is possible that the optineurin gene may cause optic nerve damage and glaucoma by promoting apoptosis of this tissue. Current studies are exploring the precise mechanism by which defects in the optineurin gene lead to glaucoma.
Section 11-C(4). Primary Congenital Glaucoma
Many cases of primary congenital glaucoma (PCG) appear to be sporadic; however, as many as 10-40% cases are familial with autosomal recessive inheritance. Many large PCG pedigrees with clear autosomal recessive inheritance have been reported. In addition, twin studies have provided strong evidence that PCG has a genetic basis. Twins with identical DNA (identical, monozygotic twins) tend to both have PCG at a much higher rate than twins with only 50% identical DNA (fraternal, dizygotic twins). This difference in concordance indicates that genes have important roles in the development of PCG.
Research studies of several large families have shown that mutations in the gene cytochrome P450 1B1 (CYP1B1) cause many cases of PCG. Most patients with PCG (87% of familial cases and 27% of sporadic cases) have glaucoma due to variations in the CYP1B1 gene.
The CYP1B1 gene encodes a protein that metabolizes or breaks down certain molecules or drugs. The mechanism by which defects in the CYP1B1 gene causes PCG is unknown. However, it has been theorized that mutations in this gene may alter its ability to break down factors that are vital to the normal development of drainage angle. A defective CYP1B1 gene might, therefore, result in an abnormal concentration of these developmental factors and lead to the abnormal formation of the drainage angle and PCG.
Section 11-D. Genetic Testing for Glaucoma
Incredible progress in being made in the field of genetic research and important discoveries and innovations are being made at an increasingly rapid pace. The Online Mendelian Inheritance in Man is a catalog of heritable diseases and syndromes. As new genes that cause various types of glaucoma are discovered, there will be more opportunities for genetic testing to enhance all aspects of patient care including diagnosis, prognosis, treatment, and family planning. Genetic testing is available for several glaucoma genes including myocilin (MYOC), optineurin (OPTN), and cytochrome P450 1B1 (CYP1B1).
Genetic testing may be useful for patients with specific types of glaucoma and particular clinical features of the disease. Patients that are interested in testing should discuss it with their physician and/or a genetic counselor. Along with discussing whether genetic testing is appropriate for a particular type of glaucoma, physicians and counselors may discuss the implications of these investigations. Genetic testing for inherited diseases may provide information that is useful for a patient’s medical care; however, this information may also affect other family members. Consequently, it is possible that genetic testing may be a source of stress or anxiety for the patient and for family members. Additionally, the results of genetic tests are complex, and should be interpreted by experienced physicians and genetic counselors. The meaning of positive or negative results is not always obvious, and must be carefully explained for genetic testing to be helpful to the patient. For example, there are likely many more glaucoma-causing genes than what have been discovered so far. If a patient is tested for defects in the known glaucoma genes (i.e. myocilin and optineurin) and no disease-causing mutations are detected, it is still possible that this patient has as yet unidentified alterations in genes that play a crucial role in glaucoma development. A patient’s physician or counselor is best equipped to explain the meaning and consequences of such genetic test results.
Some general guidelines for who may benefit most from some specific types of genetic testing are provided below.
Myocilin genetic testing for JOAG.
The patients with the highest likelihood of having glaucoma that is associated with a defect in the myocilin gene are patients with an early onset of disease (< 40 years of age); extremely high intraocular pressure (> 30 mm Hg); and a strong family history of disease. Most patients with these characteristics have familial juvenile-onset primary open angle glaucoma (JOAG) that is due to a mutation in the myocilin gene. Genetic testing may provide patients with familial JOAG and their physicians useful information to help solidify a diagnosis of this form of glaucoma.
Myocilin (MYOC) genetic testing for POAG.
Mutations in the myocilin gene account for a smaller proportion of POAG cases (3-4%) than JOAG. Due to the relatively low prevalence of myocilin-associated POAG and the labor-intensive nature of the mutation detection tests, large-scale testing of the general population for myocilin defects is not feasible. However, testing those individuals who are at extremely high risk for developing myocilin-associated POAG may be warranted. Such patients would include family members of patients with known myocilin-associated glaucoma and members of families with a strong history of inherited POAG.
Optineurin (OPTN) genetic testing for NTG.
Most cases of normal tension glaucoma (NTG) occur sporadically, without a family history of disease. However, there are rare familial cases of NTG and testing these patients for mutations in the optineurin gene may be warranted.
Cytochrome P450 1B1 (CYP1B1) testing for PCG.
Many cases of PCG are due to mutations in the CYP1B1 gene. In certain European populations of patients, as much as 87% of family cases of PCG and 27% of sporadic cases of PCG are caused by mutations of the CYP1B1 gene. However, the frequency of CYP1B1 mutations in cases of PCG in the United States is not precisely known. Based on this information, it is usually reasonable to test for CYP1B1 mutations in PCG patients with a positive family history of disease. While the likelihood of detecting a CYP1B1 mutation in a PCG patient is lower when there is no family history of disease, genetic testing may be warranted in some of these sporadic cases.
Section 11-E. The Benefits of Studying Glaucoma Genetics
Every year, thousands of Americans are blinded by glaucoma. In most cases, the loss of vision caused by glaucoma could be limited or prevented by currently available therapies if the disease were identified in its early stages. Many cases of glaucoma are not discovered until vision has already been permanently lost, because clinical signs of early glaucoma are subtle and silent to the patient.
The discovery of glaucoma disease genes provides a method for early detection of glaucoma. Genetic testing for disease-causing mutations in these genes is capable of identifying those at highest risk for developing glaucoma years to decades before vision loss or other symptoms are manifested. Heightened surveillance and early institution of glaucoma therapy can then be provided to these patients before any vision is lost, perhaps even before any symptoms are observed.
Testing to determine the genetic causes of glaucoma will also facilitate the development and evaluation of new medical therapies and surgical interventions.
Glaucoma is a collection of distinct diseases with similar clinical appearances. Genetic testing will allow physicians to identify groups of patients with the same biochemical basis of glaucoma. Some sub-types of glaucoma may respond to certain treatments while others may not. Identification of such well-characterized groups of patients to test new medical therapies and surgical interventions will help speed the discovery of new, effective treatments.
Genetic study of inherited diseases such as glaucoma will likely promote advances in therapy as well as diagnosis. For example, methods to replace defective genes with normal functioning genes (trans-genes) are being perfected. There are, however, some limitations to this technology known as gene therapy. Some of the major obstacles in using gene therapy include difficulties in obtaining 1) effective delivery of the trans-genes to the right tissues of the eye, 2) control of transgene activity, 3) maintenance of transgene effect, and 4) low-cost methodology. Advances in all of these areas are being realized, and gene therapy for glaucoma may be possible in the future.
Most inherited genetic defects, however, may be treated with currently available medical and surgical therapies. As the functions of disease-causing genes are discovered, conventional treatments may also be tailored to mitigate disease-causing defects. In one form or another, genetic research opens the promise of a new generation of sight-saving therapies
Chapter 11. References
Kwon YH, Fingert JH, Kuehn MH, Alward WLM. Mechanisms of Disease: Primary Open-Angle Glaucoma. New Engl J Med, 360:1113-24, 2009.
Fingert JH, Anderson, MG. Chapter 144: Glaucoma. In Emery and Rimoin’s Principles and Practice of Medical Genetics. 5th Ed. Elsevier, Philadelphia, 3133-3156, 2006.
Allingham RR, et al., editors. Shields’ Textbook of Glaucoma, ed. 5. Philadelphia: Lippincott Williams & Wilkins, 163-169, 2005.
Fingert JH, Stone EM, Sheffield VC, Alward WLM. Myocilin Glaucoma. Survey of Ophthalmology, 47: 547-561, 2002.
Sheffield VC, Alward WLM, Stone EM. Chapter 242: The Glaucomas. In Scriver CR, et al, eds. The Metabolic & Molecular Basis of Inherited Disease. 8th Ed. MacGraw-Hill, St. Louis, 6063-6075, 2001.
Johnson AT, Alward WLM, Sheffield VC, Stone EM. Chapter 2: Genetics and Glaucoma. In Ritch R, Shield MB, Krupin T, eds. The Glaucomas. 2nd Ed. Mosby, Chicago, 39-54, 1996
GeneReviews
The John and Marcia Carver Non-profit Genetic Testing Laboratory
http://www.carverlab.org.